Abstract
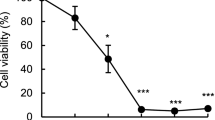
13-Oxyingenol dodecanoate (13OD) is an ingenol derivative prepared from Chinese traditional medicine Euphorbia kansui without any report about its bioactivity. The present study demonstrated for the first time that 13OD displayed potent cytotoxicity against chronic myeloid leukemia K562 cells in vitro. 13OD inhibited proliferation, induced G2/M phase arrest, and exhibited potent apoptotic activity in K562 cells. In K562 cells, 13OD disrupted the mitochondrial membrane potential and induced high level of ROS, which played an indispensable role in 13OD-induced apoptosis. Further investigations on the molecular mechanisms revealed that total Akt protein level was decreased in a caspase-dependent way after treatment with 13OD; in addition, ERK was activated by 13OD, and this activation played a protective role in 13OD stimulation. Altogether, these results revealed that the cytotoxic ingenol derivative 13OD induced apoptosis with novel mechanisms for the proapoptotic function in cancer cells, and suggested that 13OD may serve as a lead template for rational drug design and for future anticancer agent development.








Similar content being viewed by others
References
Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7(5):345–56.
Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK//STAT, Raf//MEK//ERK, PI3K//Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18(2):189–218.
Burchert A, Wang Y, Cai D, von Bubnoff N, Paschka P, Muller-Brusselbach S, et al. Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development. Leukemia. 2005;19(10):1774–82.
Wong S-F, Mirshahidi H. Use of tyrosine kinase inhibitors for chronic myeloid leukemia: management of patients and practical applications for pharmacy practitioners. Ann Pharmacother. 2011;45(6):787–97. doi:10.1345/aph.1P784.
Roychowdhury S, Talpaz M. Managing resistance in chronic myeloid leukemia. Blood Rev. 2011;25(6):279–90. doi:10.1016/j.blre.2011.09.001.
Abreu CM, Price SL, Shirk EN, Cunha RD, Pianowski LF, Clements JE et al. Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR. PLoS One. 2014;9(5).
Jiang G, Mendes EA, Kaiser P, Sankaran-Walters S, Tang Y, Weber MG, et al. Reactivation of HIV latency by a newly modified ingenol derivative via protein kinase Cdelta-NF-kappaB signaling. Aids. 2014;28(11):1555–66.
Fujiwara M, Ijichi K, Konno K, Yokota T, Tokuhisa K, Katsuura K, et al. Ingenol derivatives, ingredient of ‘Kansui’, are highly potent inhibitor of HIV. Antivir Res. 1995;26(3):A228. doi:10.1016/0166-3542(95)94704-6.
Benhadji KA, Serova M, Ghoul A, Cvitkovic E, Le Tourneau C, Ogbourne SM, et al. Antiproliferative activity of PEP005, a novel ingenol angelate that modulates PKC functions, alone and in combination with cytotoxic agents in human colon cancer cells. Br J Cancer. 2008;99(11):1808–15.
Liang X, Grue-Sorensen G, Mansson K, Vedso P, Soor A, Stahlhut M, et al. Syntheses, biological evaluation and SAR of ingenol mebutate analogues for treatment of actinic keratosis and non-melanoma skin cancer. Bioorg Med Chem Lett. 2013;23(20):5624–9.
Aditya S, Gupta S. Ingenol mebutate: a novel topical drug for actinic keratosis. Indian Dermatol Online J. 2013;4(3):246–9. doi:10.4103/2229-5178.115538.
Tzogani K, Nagercoil N, Hemmings RJ, Samir B, Gardette J, Demolis P, et al. The European medicines agency approval of ingenol mebutate (Picato) for the cutaneous treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis in adults: Summary of the scientific assessment of the committee for medicinal products for human use (CHMP). Eur J Dermatol. 2014;24(4):457–63.
Uemura D, Hirata Y, Yuh-Pan C, Hong-Yen H. New diterpene, 13-oxyingenol, derivative isolated from euphorbia kansui liou. Tetrahedron Lett. 1974;15(29):2529–32. doi:10.1016/S0040-4039(01)93197-1.
Song X, Zhao Z, Qi X, Tang S, Wang Q, Zhu T et al. Identification of epipolythiodioxopiperazines HDN-1 and chaetocin as novel inhibitor of heat shock protein 90. Oncotarget. 2015;6(7):5263–74.
Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci. 2009;122(Pt 4):437–41.
Zeng C-W, Zhang X-J, Lin K-Y, Ye H, Feng S-Y, Zhang H, et al. Camptothecin induces apoptosis in cancer cells via microRNA-125b-mediated mitochondrial pathways. Mol Pharmacol. 2012;81(4):578–86. doi:10.1124/mol.111.076794.
Timme CR, Gruidl M, Yeatman TJ. Gamma-secretase inhibition attenuates oxaliplatin-induced apoptosis through increased Mcl-1 and/or Bcl-xL in human colon cancer cells. Apoptosis. 2013;18(10):1163–74.
Hampson P, Chahal H, Khanim F, Hayden R, Mulder A, Assi LK, et al. PEP005, a selective small-molecule activator of protein kinase C, has potent antileukemic activity mediated via the delta isoform of PKC. Blood. 2005;106(4):1362–8.
Bhagatte Y, Lodwick D, Storey N. Mitochondrial ROS production and subsequent ERK phosphorylation are necessary for temperature preconditioning of isolated ventricular myocytes. Cell Death Dis. 2012;5(3):84.
Wang X, Martindale JL, Holbrook NJ. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000;275(50):39435–43.
Mann KK, Colombo M, Miller Jr WH. Arsenic trioxide decreases AKT protein in a caspase-dependent manner. Mol Cancer Ther. 2008;7(6):1680–7.
Kim T, Keum G, Pae AN. Discovery and development of heat shock protein 90 inhibitors as anticancer agents: a review of patented potent geldanamycin derivatives. Expert Opin Ther Pat. 2013;23(8):919–43.
Beck R, Dejeans N, Glorieux C, Creton M, Delaive E, Dieu M, et al. Hsp90 is cleaved by reactive oxygen species at a highly conserved N-terminal amino acid motif. PLoS One. 2012;7(7):e40795. doi:10.1371/journal.pone.0040795.
Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277(42):39858–66.
Martin D, Salinas M, Fujita N, Tsuruo T, Cuadrado A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J Biol Chem. 2002;277(45):42943–52.
Nambudiri V. From home remedy to cancer treatment: a history of ingenol mebutate and euphorbia peplus in dermatology. J Am Acad Dermatol. 2013;68(4, Supplement 1):AB33. doi:10.1016/j.jaad.2012.12.141.
Kroemer G. Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun. 2003;304(3):433–5.
Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–811. doi:10.1038/sj.onc.1209608.
Polivka Jr J, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142(2):164–75. doi:10.1016/j.pharmthera.2013.12.004.
Serova M, Ghoul A, Benhadji KA, Faivre S, Le Tourneau C, Cvitkovic E, et al. Effects of protein kinase C modulation by PEP005, a novel ingenol angelate, on mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling in cancer cells. Mol Cancer Ther. 2008;7(4):915–22.
Mas VM, Hernandez H, Plo I, Bezombes C, Maestre N, Quillet-Mary A, et al. Protein kinase Czeta mediated Raf-1/extracellular-regulated kinase activation by daunorubicin. Blood. 2003;101(4):1543–50.
Xiao RZ, He CM, Xiong MJ, Ruan XX, Wang LL, Chen Y, et al. Inhibition of extracellular signal-regulated kinase activity by sorafenib increases sensitivity to DNR in K562 cells. Oncol Rep. 2013;29(5):1895–901.
Gores GJ, Kaufmann SH. Selectively targeting Mcl-1 for the treatment of acute myelogenous leukemia and solid tumors. Genes Dev. 2012;26(4):305–11.
Kedei N, Lundberg DJ, Toth A, Welburn P, Garfield SH, Blumberg PM. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004;64(9):3243–55.
Medina EA, Afsari RR, Ravid T, Castillo SS, Erickson KL, Goldkorn T. Tumor necrosis factor-{alpha} decreases Akt protein levels in 3T3-L1 adipocytes via the caspase-dependent ubiquitination of Akt. Endocrinology. 2005;146(6):2726–35.
Acknowledgments
This work was supported by the NSFC-Shandong Joint Fund (No. U1406402), the Natural Science Foundation of China (No. 81373323), the Natural Science Foundation of the Shandong Province (No. ZR2012CM005, No. ZR2015HM010), and the Young Talent Project at Ocean University of China (No. 201412007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Liu, M., Zhang, W., Wang, G. et al. 13-Oxyingenol dodecanoate, a cytotoxic ingenol derivative, induces mitochondrial apoptosis and caspase-dependent Akt decrease in K562 cells. Tumor Biol. 37, 6227–6238 (2016). https://doi.org/10.1007/s13277-015-4495-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4495-7