Abstract
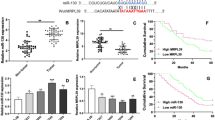
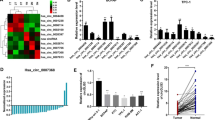
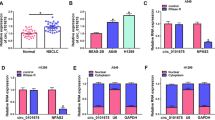
MicroRNAs (miRNAs) play key roles in cancer development and progression. In the present study, we investigated the role of miR-145 in the progression of hepatocellular carcinoma (HCC). Ten HCC cell lines and samples from 96 patients with HCC were analyzed for the expression of miR-145 by quantitative real-time polymerase chain reaction (qRT-PCR). Overexpression of miR-145 was established by transfecting mimics into HepG2 and QGY-7703 cells. Cell proliferation and cell migration were assessed by cell viability assay and transwell assay. Western blot was to verify ROCK1 as a novel target gene of miR-145. Our results showed that miR-145 was frequently downregulated in HCC tumors and cell lines. Overexpression of miR-145 in HCC cell lines significantly inhibited cell proliferation, migration, and invasion in vitro. ROCK1 was identified as a target of miR-145, and ectopic expression of miR-145 downregulated ROCK1. Together, these findings indicate that miR-145 acts as a tumor suppressor and its downregulation in tumor tissues may contribute to the progression and metastasis of HCC through a mechanism involving ROCK1, suggesting miR-145 as a potential new diagnostic and therapeutic target for the treatment of HCC.





Similar content being viewed by others
References
Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15 Suppl 4:5–13.
Teo EK, Fock KM. Hepatocellular carcinoma: an Asian perspective. Dig Dis. 2001;19:263–8.
Jonas S, Bechstein WO, Steinmuller T, Herrmann M, Radke C, Berg T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–6.
Kloosterman W, Plasterk R. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–50.
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8.
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006.
Schetter AJ, Leung SY, Sohn JJ, Zanetti KA, Bowman ED, Yanaihara N, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–36.
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8.
Lakshmipathy U, Love B, Adams C, Thyagarajan B, Chesnut JD. Micro RNA profiling: an easy and rapid method to screen and characterize stem cell populations. Methods Mol Biol. 2007;407:97–114.
Michael MZ, O’ Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–91.
Doberstein K, Steinmeyer N, Hartmetz AK, Eberhardt W, Mittelbronn M, Harter PN, et al. MicroRNA-145 targets the metalloprotease ADAM17 and is suppressed in renal cell carcinoma patients. Neoplasia. 2013;15:218–30.
Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69.
Chiyomaru T, Enokida H, Tatarano S, Kawahara K, Uchida Y, Nishiyama K, et al. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102:883–91.
Morgan-Fisher M, Wewer UM, Yoneda A. Regulation of ROCK activity in cancer. J Histochem Cytochem. 2013;61:185–98.
Ma W, Wong CC, Tung EK, Wong CM, Ng IO. RhoE is frequently downregulated in HCC and suppresses HCC invasion through antagonizing the Rho/ROCK/MYPT pathway. Hepatology. 2012;57:152–61.
Acknowledgments
This study was supported by National Natural Science Foundation of China grant (No. 81360274 and No. 31200657).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Informed consents
All specimens were collected in accordance with informed consents of patients, and all procedures complied with the protocol approved by the Ethical Committee of Kunming General Hospital.
Rights and permissions
About this article
Cite this article
Ding, W., Tan, H., Zhao, C. et al. MiR-145 suppresses cell proliferation and motility by inhibiting ROCK1 in hepatocellular carcinoma. Tumor Biol. 37, 6255–6260 (2016). https://doi.org/10.1007/s13277-015-4462-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4462-3