Abstract
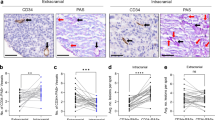
CD105 is rich in endothelium cells and is involved in angiogenesis. Higher microvascular density of tumor is also related to the prognosis in a variety of cancers. In this present study, patients with positive N classification, advanced T classification, advanced TNM stage, extracapsular spread of lymph nodes (ECS), and perineural invasion had significantly higher levels of peripheral vein (pCD105) and venous return from tumor (tCD105) in 71 patients with OSCC compared to 13 healthy volunteers. Those with higher pCD105 or tCD105 levels had significantly poorer 5-year disease-specific survival rate (DDS) and overall survival rate (OS). The tCD105 and pCD105 levels and ECS were the independent prognostic factors by the multivariate analysis according to the Cox regression model in 5-year DDS and OS rate. SAS and SCC4 cells treated with CD105 showed the increase in migration, invasion, and proliferation in vitro and in vivo. Furthermore, CCL20 expression participated in CD105-elicited cell motility in oral cancer cells. In conclusion, higher level of circulating CD105 is related to adverse pathological features among patients with OSCC. It is also a useful marker for evaluating the prognosis and targeting therapeutics of OSCC.




Similar content being viewed by others
References
Chien CY, Su CY, Chuang HC, Fang FM, Huang HY, Chen CH, et al. Comprehensive study on the prognostic role of osteopontin expression in oral squamous cell carcinoma. Oral Oncol. 2009;45(9):798–802. doi:10.1016/j.oraloncology.2008.12.006.
Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res. 1997;3(7):1031–44.
Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, et al. Endoglin (CD105): a marker of tumor vasculature and potential target for therapy. Clin Cancer Res. 2008;14(7):1931–7. doi:10.1158/1078-0432.CCR-07-4478.
Bobik A. Transforming growth factor-betas and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26(8):1712–20. doi:10.1161/01.ATV.0000225287.20034.2c.
Chuang HC, Su CY, Huang HY, Chien CY, Chen CM, Huang CC. High expression of CD105 as a prognostic predictor of early tongue cancer. Laryngoscope. 2006;116(7):1175–9. doi:10.1097/01.mlg.0000224338.56902.28.
Martone T, Rosso P, Albera R, Migliaretti G, Fraire F, Pignataro L, et al. Prognostic relevance of CD105+ microvessel density in HNSCC patient outcome. Oral Oncol. 2005;41(2):147–55. doi:10.1016/j.oraloncology.2004.08.001.
Marioni G, Marino F, Giacomelli L, Staffieri C, Mariuzzi ML, Violino E, et al. Endoglin expression is associated with poor oncologic outcome in oral and oropharyngeal carcinoma. Acta Otolaryngol. 2006;126(6):633–9. doi:10.1080/00016480500452558.
Chien CY, Su CY, Hwang CF, Chuang HC, Hsiao YC, Wu SL, et al. Clinicopathologic significance of CD105 expression in squamous cell carcinoma of the hypopharynx. Head Neck. 2006;28(5):441–6. doi:10.1002/hed.20364.
Marioni G, Staffieri A, Manzato E, Ralli G, Lionello M, Giacomelli L, et al. A higher CD105-assessed microvessel density and worse prognosis in elderly patients with laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137(2):175–80. doi:10.1001/archoto.2010.244.
Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350(24):2461–70. doi:10.1056/NEJMoa032260.
Chen CH, Chang AY, Li SH, Tsai HT, Shiu LY, Su LJ, et al. Suppression of Aurora-A-FLJ10540 signaling axis prohibits the malignant state of head and neck cancer. Mol Cancer. 2015;14:83. doi:10.1186/s12943-015-0348-7.
Calvayrac O, Rodriguez-Calvo R, Alonso J, Orbe J, Martin-Ventura JL, Guadall A, et al. CCL20 is increased in hypercholesterolemic subjects and is upregulated by LDL in vascular smooth muscle cells: role of NF-kappaB. Arterioscler Thromb Vasc Biol. 2011;31(11):2733–41. doi:10.1161/ATVBAHA.111.235721.
Hwang CF, Shiu LY, Su LJ, Yu-Fang Y, Wang WS, Huang SC, et al. Oncogenic fibulin-5 promotes nasopharyngeal carcinoma cell metastasis through the FLJ10540/AKT pathway and correlates with poor prognosis. PloS one. 2013;8(12):e84218. doi:10.1371/journal.pone.0084218.
Chen CH, Shiu LY, Su LJ, Huang CY, Huang SC, Huang CC, et al. FLJ10540 is associated with tumor progression in nasopharyngeal carcinomas and contributes to nasopharyngeal cell proliferation, and metastasis via osteopontin/CD44 pathway. J Transl Med. 2012;10:93. doi:10.1186/1479-5876-10-93.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi:10.1006/meth.2001.1262.
Yang TY, Chiang NY, Tseng WY, Pan HL, Peng YM, Shen JJ, et al. Expression and immunoaffinity purification of recombinant soluble human GPR56 protein for the analysis of GPR56 receptor shedding by ELISA. Protein Expr Purif. 2015;109:85–92. doi:10.1016/j.pep.2014.11.013.
Chen CH, Chuang HC, Huang CC, Fang FM, Huang HY, Tsai HT, et al. Overexpression of Rap-1A indicates a poor prognosis for oral cavity squamous cell carcinoma and promotes tumor cell invasion via Aurora-A modulation. Am J Pathol. 2013;182(2):516–28. doi:10.1016/j.ajpath.2012.10.023.
Chien CY, Tsai HT, Su LJ, Chuang HC, Shiu LY, Huang CC, et al. Aurora-A signaling is activated in advanced stage of squamous cell carcinoma of head and neck cancer and requires osteopontin to stimulate invasive behavior. Oncotarget. 2014;5(8):2243–62.
Hong DY, Lee BJ, Lee JC, Choi JS, Wang SG, Ro JH. Expression of VEGF, HGF, IL-6, IL-8, MMP-9, Telomerase in Peripheral Blood of Patients with Head and Neck Squamous Cell Carcinoma. Clin Exp Otorhinolaryngol. 2009;2(4):186–92. doi:10.3342/ceo.2009.2.4.186.
Druzgal CH, Chen Z, Yeh NT, Thomas GR, Ondrey FG, Duffey DC, et al. A pilot study of longitudinal serum cytokine and angiogenesis factor levels as markers of therapeutic response and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2005;27(9):771–84. doi:10.1002/hed.20246.
Teknos TN, Cox C, Yoo S, Chepeha DB, Wolf GT, Bradford CR, et al. Elevated serum vascular endothelial growth factor and decreased survival in advanced laryngeal carcinoma. Head Neck. 2002;24(11):1004–11. doi:10.1002/hed.10163.
Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17(9):984–92. doi:10.1096/fj.02-0634rev.
Li C, Gardy R, Seon BK, Duff SE, Abdalla S, Renehan A, et al. Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer. 2003;88(9):1424–31. doi:10.1038/sj.bjc.6600874.
Li C, Guo B, Wilson PB, Stewart A, Byrne G, Bundred N, et al. Plasma levels of soluble CD105 correlate with metastasis in patients with breast cancer. Int J Cancer J Int du Cancer. 2000;89(2):122–6.
Svatek RS, Karam JA, Roehrborn CG, Karakiewicz PI, Slawin KM, Shariat SF. Preoperative plasma endoglin levels predict biochemical progression after radical prostatectomy. Clin Cancer Res. 2008;14(11):3362–6. doi:10.1158/1078-0432.CCR-07-4707.
Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, et al. Endoglin promotes endothelial cell proliferation and TGF-beta/ALK1 signal transduction. EMBO J. 2004;23(20):4018–28. doi:10.1038/sj.emboj.7600386.
Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, et al. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31(6):2283–90.
Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, et al. Defective angiogenesis in mice lacking endoglin. Science. 1999;284(5419):1534–7.
Burrows FJ, Derbyshire EJ, Tazzari PL, Amlot P, Gazdar AF, King SW, et al. Up-regulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res. 1995;1(12):1623–34.
Paauwe M, ten Dijke P, Hawinkels LJ. Endoglin for tumor imaging and targeted cancer therapy. Expert Opin Ther Targets. 2013;17(4):421–35. doi:10.1517/14728222.2013.758716.
Nolan-Stevaux O, Zhong W, Culp S, Shaffer K, Hoover J, Wickramasinghe D, et al. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti-endoglin antibodies. PloS one. 2012;7(12):e50920. doi:10.1371/journal.pone.0050920.
Rosen LS, Hurwitz HI, Wong MK, Goldman J, Mendelson DS, Figg WD, et al. A phase I first-in-human study of TRC105 (Anti-Endoglin Antibody) in patients with advanced cancer. Clin Cancer Res. 2012;18(17):4820–9. doi:10.1158/1078-0432.CCR-12-0098.
Jonker L. TGF-β & BMP receptors endoglin and ALK1: overview of their functional role and status as anti-angiogenic targets. Microcirculation. 2013;Accepted manuscript online. doi:10.1111/micc.12099.
Choi KS, Bae MK, Jeong JW, Moon HE, Kim KW. Hypoxia-induced angiogenesis during carcinogenesis. J Biochem Mol Biol. 2003;36(1):120–7.
Tian F, Zhou AX, Smits AM, Larsson E, Goumans MJ, Heldin CH, et al. Endothelial cells are activated during hypoxia via endoglin/ALK-1/SMAD1/5 signaling in vivo and in vitro. Biochem Biophys Res Commun. 2010;392(3):283–8. doi:10.1016/j.bbrc.2009.12.170.
Sanz-Rodriguez F, Fernandez LA, Zarrabeitia R, Perez-Molino A, Ramirez JR, Coto E, et al. Mutation analysis in Spanish patients with hereditary hemorrhagic telangiectasia: deficient endoglin up-regulation in activated monocytes. Clin Chem. 2004;50(11):2003–11. doi:10.1373/clinchem.2004.035287.
Torsney E, Charlton R, Parums D, Collis M, Arthur HM. Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm Res. 2002;51(9):464–70.
Scharpfenecker M, Floot B, Russell NS, Stewart FA. The TGF-beta co-receptor endoglin regulates macrophage infiltration and cytokine production in the irradiated mouse kidney. Radiother Oncol. 2012;105(3):313–20. doi:10.1016/j.radonc.2012.08.021.
Wolff HA, Rolke D, Rave-Frank M, Schirmer M, Eicheler W, Doerfler A, et al. Analysis of chemokine and chemokine receptor expression in squamous cell carcinoma of the head and neck (SCCHN) cell lines. Radiat Environ Biophys. 2011;50(1):145–54. doi:10.1007/s00411-010-0341-x.
Ding X, Wang K, Wang H, Zhang G, Liu Y, Yang Q, et al. High expression of CCL20 is associated with poor prognosis in patients with hepatocellular carcinoma after curative resection. J Gastrointest Surg. 2012;16(4):828–36. doi:10.1007/s11605-011-1775-4.
Acknowledgments
This study was supported in part by grants CMRPG890091-3, CMRPG8B0971-2, CMRPG8D1421, CMRPG890921, CMRPG8A0391-2, CMRPG8C0591-2, CMRPG8B1251-3, CMRPG8C0581-2, and CMRPG8B1451 from the Chang Gung Memorial Hospital and the grants MOST-98-2314-B-182A-042-MY3, MSOT-101-2314-B-182A-043-MY3, MOST-104-2314-B-182A-075-MY3, MOST-103-2320-B-182A-015-, and MOST-104-2320-B-182A-010- from the Ministry of Science and Technology of Taiwan. We would also like to thank the Chang Gung Medical Foundation Kaohsiung Chang Gung Memorial Hospital Tissue Bank (CLRPG870463) for technical support.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contributions
Chang-Han Chen, Hui-Ching Chuang, Yu-Tsai Lin, and Chih-Yen Chien collected the clinical data of patients, conceived the study design, carried out and coordinated immunohistochemical examinations of tumor specimens and data analysis, and drafted the manuscript. Fu-Min Fang and Hui Lu performed statistical data analysis. Chao-Cheng Huang participated in the interpretation of data and conducted immunohistochemistry analysis. Ching-Mei Chen carried out immunohistochemical examinations of tumor specimens.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, CH., Chuang, HC., Lin, YT. et al. Circulating CD105 shows significant impact in patients of oral cancer and promotes malignancy of cancer cells via CCL20. Tumor Biol. 37, 1995–2005 (2016). https://doi.org/10.1007/s13277-015-3991-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3991-0