Abstract
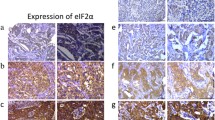
Altered expression of eukaryotic translation initiation factor 5A-2 (eIF5A-2) was associated with human carcinogenesis and progression. This study assessed eIF5A-2 expression in gastric cancer tissues for association with clinicopathological parameters and survival of patients. A total of 436 gastric cancer tissues and 92 normal mucosal blocks were collected for construction of tissue microarrays and immunohistochemical assessment of eIF5A-2 expression. The data were statistically analyzed for association with clinicopathological factors and survival of patients. Immunohistochemical data showed that eIF5A-2 protein was highly expressed in gastric cancer tissues (p < 0.001). Upregulated expression of eIF5A-2 protein was associated with tumor Lauren classification, size, location, invasion, TNM stages, and lymph node and distant metastases. The 3- and 5-year cumulative survival rates of these 436 patients were 88.5 and 58.1 %, respectively. In contrast, the mean survival time of patients with increased tumor eIF5A-2 was 30.22 ± 1.23 vs. 51.29 ± 0.86 months for those with low tumor eIF5A-2 (p < 0.001). Multivariate analysis showed that eIF5A-2 expression and related tumor parameters were independent indicators of overall survival in gastric cancer patients. In conclusion, the current study indicates that overexpression of eIF5A-2 protein was associated with poor overall survival of gastric cancer patients.


Similar content being viewed by others
References
Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from major cancers in the European Union, including acceding countries, in 2004. Cancer. 2004;101(12):2843–50. doi:10.1002/cncr.20666.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354–62.
2014 WCR.
Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–49. doi:10.3748/wjg.v20.i7.1635.
Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al. EUROCARE-3: survival of cancer patients diagnosed 1990–94—results and commentary. Ann Oncol. 2003;14 Suppl 5:v61–118.
Grabsch HI, Tan P. Gastric cancer pathology and underlying molecular mechanisms. Dig Surg. 2013;30(2):150–8. doi:10.1159/000350876.
Li J, Davies BR, Han S, Zhou M, Bai Y, Zhang J, et al. The AKT inhibitor AZD5363 is selectively active in PI3KCA mutant gastric cancer, and sensitizes a patient-derived gastric cancer xenograft model with PTEN loss to Taxotere. J Transl Med. 2013;11:241. doi:10.1186/1479-5876-11-241.
Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11(6):3105–14.
Park MH. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J Biochem. 2006;139(2):161–9. doi:10.1093/jb/mvj034.
Clement PM, Johansson HE, Wolff EC, Park MH. Differential expression of eIF5A-1 and eIF5A-2 in human cancer cells. Febs J. 2006;273(6):1102–14. doi:10.1111/j.1742-4658.2006.05135.x.
Clement PM, Henderson CA, Jenkins ZA, Smit-McBride Z, Wolff EC, Hershey JW, et al. Identification and characterization of eukaryotic initiation factor 5A-2. Eur J Biochem. 2003;270(21):4254–63.
Jenkins ZA, Haag PG, Johansson HE. Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved vertebrate variant of eukaryotic translation initiation factor 5A with tissue-specific expression. Genomics. 2001;71(1):101–9. doi:10.1006/geno.2000.6418.
Chen W, Luo JH, Hua WF, Zhou FJ, Lin MC, Kung HF, et al. Overexpression of EIF-5A2 is an independent predictor of outcome in patients of urothelial carcinoma of the bladder treated with radical cystectomy. Cancer Epidemiol Biomarkers Prev. 2009;18(2):400–8. doi:10.1158/1055-9965.EPI-08-0754.
Gosslau A, Jao DL, Butler R, Liu AY, Chen KY. Thermal killing of human colon cancer cells is associated with the loss of eukaryotic initiation factor 5A. J Cell Physiol. 2009;219(2):485–93. doi:10.1002/jcp.21696.
Guan XY, Sham JS, Tang TC, Fang Y, Huo KK, Yang JM. Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res. 2001;61(9):3806–9.
Lee HS, Cho SB, Lee HE, Kim MA, Kim JH, do Park J, et al. Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res. 2007;13(14):4154–63. doi:10.1158/1078-0432.CCR-07-0173.
Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003;200(1):39–46. doi:10.1002/path.1288.
Geisler SA, Olshan AF, Weissler MC, Cai J, Funkhouser WK, Smith J, et al. p16 and p53 protein expression as prognostic indicators of survival and disease recurrence from head and neck cancer. Clin Cancer Res. 2002;8(11):3445–53.
Song LB, Liao WT, Mai HQ, Zhang HZ, Zhang L, Li MZ, et al. The clinical significance of twist expression in nasopharyngeal carcinoma. Cancer Lett. 2006;242(2):258–65. doi:10.1016/j.canlet.2005.11.013.
Wu HH, Lin WC, Tsai KW. Advances in molecular biomarkers for gastric cancer: miRNAs as emerging novel cancer markers. Expert Rev Mol Med. 2014;16, e1. doi:10.1017/erm.2013.16.
Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4(8):657–65. doi:10.1038/nrm1175.
Kawakami H, Okamoto I, Arao T, Okamoto W, Matsumoto K, Taniguchi H, et al. MET amplification as a potential therapeutic target in gastric cancer. Oncotarget. 2013;4(1):9–17.
Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu L, et al. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology. 2010;51(4):1255–63. doi:10.1002/hep.23451.
Hu L, Wen JM, Sham JS, Wang W, Xie D, Tjia WM, et al. Establishment of cell lines from a primary hepatocellular carcinoma and its metastatis. Cancer Genet Cytogenet. 2004;148(1):80–4.
Petersen I, Bujard M, Petersen S, Wolf G, Goeze A, Schwendel A, et al. Patterns of chromosomal imbalances in adenocarcinoma and squamous cell carcinoma of the lung. Cancer Res. 1997;57(12):2331–5.
Shek FH, Fatima S, Lee NP. Implications of the use of eukaryotic translation initiation factor 5A (eIF5A) for prognosis and treatment of hepatocellular carcinoma. Int J Hepatol. 2012;2012:760928. doi:10.1155/2012/760928.
Xie D, Ma NF, Pan ZZ, Wu HX, Liu YD, Wu GQ, et al. Overexpression of EIF-5A2 is associated with metastasis of human colorectal carcinoma. Hum Pathol. 2008;39(1):80–6. doi:10.1016/j.humpath.2007.05.011.
Yang GF, Xie D, Liu JH, Luo JH, Li LJ, Hua WF, et al. Expression and amplification of eIF-5A2 in human epithelial ovarian tumors and overexpression of EIF-5A2 is a new independent predictor of outcome in patients with ovarian carcinoma. Gynecol Oncol. 2009;112(2):314–8. doi:10.1016/j.ygyno.2008.10.024.
Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, et al. An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135(5):852–64. doi:10.1016/j.cell.2008.09.061.
Lee NP, Tsang FH, Shek FH, Mao M, Dai H, Zhang C, et al. Prognostic significance and therapeutic potential of eukaryotic translation initiation factor 5A (eIF5A) in hepatocellular carcinoma. Int J Cancer. 2010;127(4):968–76. doi:10.1002/ijc.25100.
He LR, Zhao HY, Li BK, Liu YH, Liu MZ, Guan XY, et al. Overexpression of eIF5A-2 is an adverse prognostic marker of survival in stage I non-small cell lung cancer patients. Int J Cancer. 2011;129(1):143–50. doi:10.1002/ijc.25669.
Marchet A, Mocellin S, Belluco C, Ambrosi A, DeMarchi F, Mammano E, et al. Gene expression profile of primary gastric cancer: towards the prediction of lymph node status. Ann Surg Oncol. 2007;14(3):1058–64. doi:10.1245/s10434-006-9090-0.
Xu GD, Shi XB, Sun LB, Zhou QY, Zheng DW, Shi HS, et al. Down-regulation of eIF5A-2 prevents epithelial-mesenchymal transition in non-small-cell lung cancer cells. J Zhejiang Univ Sci B. 2013;14(6):460–7. doi:10.1631/jzus.B1200200.
Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ, Liao YJ, et al. Ablation of EIF5A2 induces tumor vasculature remodeling and improves tumor response tochemotherapy via regulation of matrix metalloproteinase 2 expression. Oncotarget. 2014;5(16):6716–33.
Khosravi S, Wong RP, Ardekani GS, Zhang G, Martinka M, Ong CJ, et al. Role of EIF5A2, a downstream target of Akt, in promoting melanoma cell invasion. Br J Cancer. 2014;110(2):399–408. doi:10.1038/bjc.2013.688.
Wei JH, Cao JZ, Zhang D, Liao B, Zhong WM, Lu J, et al. EIF5A2 predicts outcome in localised invasive bladder cancer and promotes bladder cancer cellaggressiveness in vitro and in vivo. Br J Cancer. 2014;110(7):1767–77. doi:10.1038/bjc.2014.52.
Chen M, Huang JD, Deng HK, Dong S, Deng W, Tsang SL,et al. Overexpression of eIF-5A2 in mice causes accelerated organismal aging by increasing chromosome instability. BMC Cancer. 2011;11:199. doi:10.1186/1471-2407-11-199.
Acknowledgments
This study was supported in part by grants from the Zhejiang Provincial Department of Science and Technology Research Foundation (no. 2008C33040) and the Zhejiang Provincial Medical Science Research Foundation (no. 2007A013). The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Compliance with ethical standards
ᅟ
Conflicts of interest
None
Research involving human participants and/or animals
This study was approved by the Ethical Committee of Zhejiang Provincial People’s Hospital.
Informed consent
Written informed consent for use of the resected samples was obtained from each patient.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, Q., Ye, Z., Zhang, Q. et al. Expression of eukaryotic translation initiation factor 5A-2 (eIF5A-2) associated with poor survival in gastric cancer. Tumor Biol. 37, 1189–1195 (2016). https://doi.org/10.1007/s13277-015-3894-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3894-0