Abstract
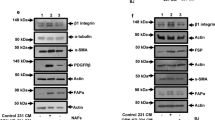
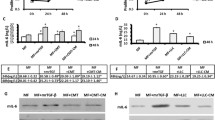
Myofibroblasts play a critical role in the cancer cell growth, invasion, and tumor-associated vascularization during the carcinogenesis of nonsmall cell lung cancer (NSCLC), whereas the underlying molecular bases are not completely understood. We isolated Lin-negative, Sca1-low, and CD49e-high myofibroblasts from the NSCLC tissues of the patients and modified the levels of either transforming growth factor β 1 (TGFβ1) or vascular endothelial growth factor A (VEGF-A) in these cells. We found that coculture with TGFβ1-overexpressing myofibroblasts significantly decreased the NSCLC cell growth in an MTT assay through proliferation suppression rather than modulation of cell apoptosis, while significantly increased the NSCLC cell invasiveness in either a transwell migration assay or a scratch wound healing migration assay. However, modulation of TGFβ1 levels in myofibroblasts did not significantly alter vessel formation in a human umbilical vein endothelial cells (HUVECs) transwell collagen gel assay. On the other hand, overexpression of VEGF-A in myofibroblasts significantly increased vessel formation in the HUVECs transwell collagen gel assay. Together, these data suggest that myofibroblasts may regulate cancer cell growth and invasion through TGFβ1 but modulate cancer-associated neovascularization through VEGF-A. Hence, targeting different signaling pathways in myofibroblasts may delicately control NSCLC growth and invasion.







Similar content being viewed by others
References
Jian H, Zhao Y, Liu B, Lu S. Sema4b inhibits growth of non-small cell lung cancer in vitro and in vivo. Cell Signal. 2015;27:1208–13.
Jian H, Zhao Y, Liu B, Lu S. Sema4b inhibits mmp9 to prevent metastasis of non-small cell lung cancer. Tumour Biol. 2014;35:11051–6.
Liu G, Xu S, Jiao F, Ren T, Li Q. Vascular endothelial growth factor b coordinates metastasis of non-small cell lung cancer. Tumour Biol. 2015;36:2185–91.
Lv Q, Shen R, Wang J. Rbpj inhibition impairs the growth of lung cancer. Tumour Biol. 2015;36:3751–6.
Pei J, Lou Y, Zhong R, Han B. Mmp9 activation triggered by epidermal growth factor induced foxo1 nuclear exclusion in non-small cell lung cancer. Tumour Biol. 2014;35:6673–8.
Wang W, Wu X, Tian Y. Crosstalk of ap4 and tgfbeta receptor signaling in nsclc. Tumour Biol. 2015;36:447–52.
Zhao D, Lu Y, Yang C, Zhou X, Xu Z. Activation of fgf receptor signaling promotes invasion of non-small-cell lung cancer. Tumour Biol. 2015;36:3637–42.
Karvonen HM, Lehtonen ST, Sormunen RT, Lappi-Blanco E, Skold CM, Kaarteenaho RL. Lung cancer-associated myofibroblasts reveal distinctive ultrastructure and function. J Thorac Oncol. 2014;9:664–74.
Karki S, Surolia R, Hock TD, Guroji P, Zolak JS, Duggal R, et al. Wilms’ tumor 1 (wt1) regulates pleural mesothelial cell plasticity and transition into myofibroblasts in idiopathic pulmonary fibrosis. FASEB J. 2014;28:1122–31.
Shu H, Li HF. Prognostic effect of stromal myofibroblasts in lung adenocarcinoma. Neoplasma. 2012;59:658–61.
Kishaba Y, Matsubara D, Niki T. Heterogeneous expression of nestin in myofibroblasts of various human tissues. Pathol Int. 2010;60:378–85.
Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–5.
Tokunou M, Niki T, Eguchi K, Iba S, Tsuda H, Yamada T, et al. C-met expression in myofibroblasts: role in autocrine activation and prognostic significance in lung adenocarcinoma. Am J Pathol. 2001;158:1451–63.
Hao Y, Zhang L, He J, Guo Z, Ying L, Xu Z, et al. Functional investigation of nci-h460-inducible myofibroblasts on the chemoresistance to vp-16 with a microfluidic 3d co-culture device. PLoS One. 2013;8:e61754.
Massague J. Tgfbeta in cancer. Cell. 2008;134:215–30.
Padua D, Massague J. Roles of tgfbeta in metastasis. Cell Res. 2009;19:89–102.
Xiao X, Wiersch J, El-Gohary Y, Guo P, Prasadan K, Paredes J, et al. Tgfbeta receptor signaling is essential for inflammation-induced but not beta-cell workload-induced beta-cell proliferation. Diabetes. 2013;62:1217–26.
Antonarakis ES, Carducci MA. Targeting angiogenesis for the treatment of prostate cancer. Expert Opin Ther Targets. 2012;16:365–76.
Kluetz PG, Figg WD, Dahut WL. Angiogenesis inhibitors in the treatment of prostate cancer. Expert Opin Pharmacother. 2010;11:233–47.
Aragon-Ching JB, Dahut WL. Vegf inhibitors and prostate cancer therapy. Curr Mol Pharmacol. 2009;2:161–8.
Delongchamps NB, Peyromaure M. The role of vascular endothelial growth factor in kidney and prostate cancer. Can J Urol. 2007;14:3669–77.
Delongchamps NB, Peyromaure M, Dinh-Xuan AT. Role of vascular endothelial growth factor in prostate cancer. Urology. 2006;68:244–8.
Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789–91.
Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood Cells Mol Dis. 2007;38:258–68.
Nieves BJ, D'Amore PA, Bryan BA. The function of vascular endothelial growth factor. Biofactors. 2009;35:332–7.
Xiao X, Prasadan K, Guo P, El-Gohary Y, Fischbach S, Wiersch J, et al. Pancreatic duct cells as a source of vegf in mice. Diabetologia. 2014;57:991–1000.
Xiao X, Guo P, Chen Z, El-Gohary Y, Wiersch J, Gaffar I, et al. Hypoglycemia reduces vascular endothelial growth factor a production by pancreatic beta cells as a regulator of beta cell mass. J Biol Chem. 2013;288:8636–46.
Donnem T, Al-Shibli K, Al-Saad S, Delghandi MP, Busund LT, Bremnes RM. Vegf-a and vegfr-3 correlate with nodal status in operable non-small cell lung cancer: inverse correlation between expression in tumor and stromal cells. Lung Cancer. 2009;63:277–83.
Sun LX, Li WD, Lin ZB, Duan XS, Li XF, Yang N, et al. Protection against lung cancer patient plasma-induced lymphocyte suppression by ganoderma lucidum polysaccharides. Cell Physiol Biochem. 2014;33:289–99.
Cao ZX, Zheng RL, Lin HJ, Luo SD, Zhou Y, Xu YZ, et al. Sklb610: a novel potential inhibitor of vascular endothelial growth factor receptor tyrosine kinases inhibits angiogenesis and tumor growth in vivo. Cell Physiol Biochem. 2011;27:565–74.
Kim HA, Seo KH, Kang YR, Ko HM, Kim KJ, Back HK, et al. Mechanisms of platelet-activating factor-induced enhancement of vegf expression. Cell Physiol Biochem. 2011;27:55–62.
Song N, Liu B, Wu J, Zhang R, Duan L, He W, et al. Vascular endothelial growth factor (vegf) -2578c/a and -460c/t gene polymorphisms and lung cancer risk: a meta-analysis involving 11 case-control studies. Tumour Biol. 2014;35:859–70.
Shi X, Liang W, Yang W, Xia R, Song Y. Decorin is responsible for progression of non-small-cell lung cancer by promoting cell proliferation and metastasis. Tumour Biol. 2015;36:3345–54.
Hao S, Yang Y, Liu Y, Yang S, Wang G, Xiao J, et al. Jam-c promotes lymphangiogenesis and nodal metastasis in non-small cell lung cancer. Tumour Biol. 2014;35:5675–87.
Gu ZZ. T, Fu BH, Hua HX, Yang S, Zhang YQ, Gao LM, Li P: Relationship of serum levels of vegf and tgf-beta1 with radiosensitivity of elderly patients with unresectable non-small cell lung cancer. Tumour Biol. 2014;35:4785–9.
Fu BH, Fu ZZ, Meng W, Gu T, Sun XD, Zhang Z (2015) Platelet vegf and serum tgf-beta1 levels predict chemotherapy response in non-small cell lung cancer patients. Tumour Biol
Ding L, Liu K, Jiang Z, Chen Q, Zhou N, Liang Y, et al. The efficacy and safety of pemetrexed plus bevacizumab in previously treated patients with advanced non-squamous non-small cell lung cancer (ns-nsclc). Tumour Biol. 2015;36:2491–9.
Deng ZC, Cao C, Yu YM, Ma HY, Ye M. Vascular endothelial growth factor -634g/c and vascular endothelial growth factor -2578c/a polymorphisms and lung cancer risk: a case-control study and meta-analysis. Tumour Biol. 2014;35:1805–11.
Akamatsu T, Arai Y, Kosugi I, Kawasaki H, Meguro S, Sakao M, et al. Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair. 2013;6:15.
Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant 81401880), Funds from Shanghai government for talent development (Grant 201455), Medical-Engineering Joint Funds from Shanghai Jiao Tong University (Grant No. YG2013MS11), and Funds from Shanghai Chest Hospital [YZ13-15].
Conflict of interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jia Huang and Ziming Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, J., Li, Z., Ding, Z. et al. Different roles of myofibroblasts in the tumorigenesis of nonsmall cell lung cancer. Tumor Biol. 37, 15525–15534 (2016). https://doi.org/10.1007/s13277-015-3862-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3862-8