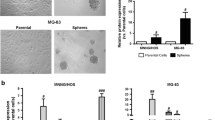
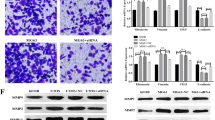
Abstract
Metformin is an oral drug that has been widely used to treat type 2 diabetes mellitus. Interestingly, accumulated evidence indicate that metformin may reduce the risk of cancer in patients with type 2 diabetes and inhibit tumor cell growth and survival in numerous malignancies, including osteosarcoma (OS) cells. In the present study, we aimed to investigate the effects of metformin on the proliferation, migration, invasion, and sphere formation in OS MG63 cells in vitro. Metformin suppressed OS MG63 cell proliferation in a dose- and time-dependent manner and markedly blocked anti-metastatic potentials, migration, and invasion, by downregulating matrix metalloproteinase 2 (MMP2) and MMP9. Besides, we established OS cancer stem-like cell (CSC) model with sarcosphere formation assay and demonstrated that metformin posed damage on CSCs in OS by inhibiting sphere formation and by inducing their stemness loss. The stemness of CSCs in OS such as self-renewal and differentiation potentials was both impaired with a significant decrease of Oct-4 and Nanog activation. Consistent with this, the positive rates of CD90, CD133, and stage-specific embryonic antigen-4 (SSEA-4) were all observed with reductions in response to metformin exposure. In addition, Western blot showed that metformin activated AMPKα at Tyr172, followed by a downregulated phosphorylation of mammalian target of rapamycin (mTOR)/S6 and feedback activation of p-AKT Ser473 in both OS MG63 cells and CSCs. This indicates that AMPK/mTOR/S6 signaling pathway might be involved in the growth inhibition of both OS MG63 cells and CSCs. These results suggest that metformin, a potential anti-neoplastic agent, might make it a novel therapeutic choice for the treatment of OS in the future.






Similar content being viewed by others
References
Arndt CA, Rose PS, Folpe AL, Laack NN. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475–87.
Bacci G, Ferrari S, Longhi A, Perin S, Forni C, Fabbri N, et al. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37:32–8.
Kempf-Bielack B, Bielack SS, Jurgens H, Branscheid D, Berdel WE, Exner GU, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol. 2005;23:559–68.
Gorlick R, Anderson P, Andrulis I, Arndt C, Beardsley GP, Bernstein M, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin Cancer Res. 2003;9:5442–53.
Chen K, Zhang S, Ji Y, Li J, An P, Ren H, et al. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS One. 2013;8, e72927.
Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan J, et al. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int J Oncol. 2015.
Wang HY, Tu YS, Long J, Zhang HQ, Qi CL, Xie XB, et al. SRF-miR29b-MMP2 axis inhibits NSCLC invasion and metastasis. Int J Oncol. 2015.
Lacorte LM, Rinaldi JC, Justulin LJ, Delella FK, Moroz A, Felisbino SL. Cadmium exposure inhibits MMP2 and MMP9 activities in the prostate and testis. Biochem Biophys Res Commun. 2015;457:538–41.
Wang XF, Wang J. Icaritin suppresses the proliferation of human osteosarcoma cells in vitro by increasing apoptosis and decreasing MMP expression. Acta Pharmacol Sin. 2014;35:531–9.
Alakus H, Grass G, Hennecken JK, Bollschweiler E, Schulte C, Drebber U, et al. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23:917–23.
Guan X, Wang X, Luo H, Wu J, Zhang X, Wu J. Matrix metalloproteinase 1, 3, and 9 polymorphisms and esophageal squamous cell carcinoma risk. Med Sci Monit. 2014;20:2269–74.
Guo J, Xu Y, Ji W, Song L, Dai C, Zhan L. Effects of exposure to benzo[a]pyrene on metastasis of breast cancer are mediated through ROS-ERK-MMP9 axis signaling. Toxicol Lett. 2015;234:201–10.
Marshall DC, Lyman SK, McCauley S, Kovalenko M, Spangler R, Liu C, et al. Selective allosteric inhibition of MMP9 is efficacious in preclinical models of ulcerative colitis and colorectal cancer. PLoS One. 2015;10, e127063.
Liu T, Zhou W, Zhang F, Shi G, Teng H, Xiao J, et al. Knockdown of IRX2 inhibits osteosarcoma cell proliferation and invasion by the AKT/MMP9 signaling pathway. Mol Med Rep. 2014;10:169–74.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–61.
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44.
Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–76.
Mahler RJ. Metformin: Actions and indications for use in non-insulin-dependent diabetes mellitus. Endocr Pract. 1995;1:418–22.
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–85.
Tsai MJ, Yang CJ, Kung YT, Sheu CC, Shen YT, Chang PY, et al. Metformin decreases lung cancer risk in diabetic patients in a dose-dependent manner. Lung Cancer. 2014;86:137–43.
Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24:469–80.
Shank JJ, Yang K, Ghannam J, Cabrera L, Johnston CJ, Reynolds RK, et al. Metformin targets ovarian cancer stem cells in vitro and in vivo. Gynecol Oncol. 2012;127:390–7.
Gou S, Cui P, Li X, Shi P, Liu T, Wang C. Low concentrations of metformin selectively inhibit CD133(+) cell proliferation in pancreatic cancer and have anticancer action. PLoS One. 2013;8, e63969.
Yang FQ, Wang JJ, Yan JS, Huang JH, Li W, Che JP, et al. Metformin inhibits cell growth by upregulating microRNA-26a in renal cancer cells. Int J Clin Exp Med. 2014;7:3289–96.
Carmignani M, Volpe AR, Aldea M, Soritau O, Irimie A, Florian IS, et al. Glioblastoma stem cells: a new target for metformin and arsenic trioxide. J Biol Regul Homeost Agents. 2014;28:1–15.
Bao B, Azmi AS, Ali S, Zaiem F, Sarkar FH. Metformin may function as anti-cancer agent via targeting cancer stem cells: the potential biological significance of tumor-associated miRNAs in breast and pancreatic cancers. Ann Transl Med. 2014;2:59.
Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–80.
Quattrini I, Conti A, Pazzaglia L, Novello C, Ferrari S, Picci P, et al. Metformin inhibits growth and sensitizes osteosarcoma cell lines to cisplatin through cell cycle modulation. Oncol Rep. 2014;31:370–5.
Duo J, Ma Y, Wang G, Han X, Zhang C. Metformin synergistically enhances antitumor activity of histone deacetylase inhibitor trichostatin a against osteosarcoma cell line. DNA Cell Biol. 2013;32:156–64.
Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep. 2009;21:1323–33.
Zhang T, Wang X, He D, Jin X, Guo P. Metformin sensitizes human bladder cancer cells to TRAIL-induced apoptosis through mTOR/S6K1-mediated downregulation of c-FLIP. Anticancer Drugs. 2014;25:887–97.
Han G, Gong H, Wang Y, Guo S, Liu K. AMPK/mTOR-mediated inhibition of survivin partly contributes to metformin-induced apoptosis in human gastric cancer cell. Cancer Biol Ther. 2015;16:77–87.
Zi FM, He JS, Li Y, Wu C, Yang L, Yang Y, et al. Metformin displays anti-myeloma activity and synergistic effect with dexamethasone in in vitro and in vivo xenograft models. Cancer Lett. 2015;356:443–53.
Lengyel E, Litchfield LM, Mitra AK, Nieman KM, Mukherjee A, Zhang Y, et al. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am J Obstet Gynecol. 2014.
Hsieh SC, Tsai JP, Yang SF, Tang MJ, Hsieh YH. Metformin inhibits the invasion of human hepatocellular carcinoma cells and enhances the chemosensitivity to sorafenib through a downregulation of the ERK/JNK-mediated NF-kappaB-dependent pathway that reduces uPA and MMP-9 expression. Amino Acids. 2014;46:2809–22.
Jang SY, Kim A, Kim JK, Kim C, Cho YH, Kim JH, et al. Metformin inhibits tumor cell migration via down-regulation of MMP9 in tamoxifen-resistant breast cancer cells. Anticancer Res. 2014;34:4127–34.
Sun XJ, Zhang P, Li HH, Jiang ZW, Jiang CC, Liu H. Cisplatin combined with metformin inhibits migration and invasion of human nasopharyngeal carcinoma cells by regulating E-cadherin and MMP-9. Asian Pac J Cancer Prev. 2014;15:4019–23.
Fang Z, Xu X, Zhou Z, Xu Z, Liu Z. Effect of metformin on apoptosis, cell cycle arrest migration and invasion of A498 cells. Mol Med Rep. 2014;9:2251–6.
Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23.
Gassmann P, Haier J, Schluter K, Domikowsky B, Wendel C, Wiesner U, et al. CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia. 2009;11:651–61.
Chen X, Guo J, Xi RX, Chang YW, Pan FY, Zhang XZ. MiR-210 expression reverses radioresistance of stem-like cells of oesophageal squamous cell carcinoma. World J Clin Oncol. 2014;5:1068–77.
So JY, Lin JJ, Wahler J, Liby KT, Sporn MB, Suh N. A synthetic triterpenoid CDDO-Im inhibits tumorsphere formation by regulating stem cell signaling pathways in triple-negative breast cancer. PLoS One. 2014;9, e107616.
Chang Y, Zhao Y, Zhan H, Wei X, Liu T, Zheng B. Bufalin inhibits the differentiation and proliferation of human osteosarcoma cell line hMG63-derived cancer stem cells. Tumour Biol. 2014;35:1075–82.
El-Merahbi R, Liu YN, Eid A, Daoud G, Hosry L, Monzer A, et al. Berberis libanotica Ehrenb extract shows anti-neoplastic effects on prostate cancer stem/progenitor cells. PLoS One. 2014;9, e112453.
Chambers I. The molecular basis of pluripotency in mouse embryonic stem cells. Cloning Stem Cells. 2004;6:386–91.
Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55.
Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–42.
Rice S, Pellat L, Ahmetaga A, Bano G, Mason HD, Whitehead SA. Dual effect of metformin on growth inhibition and oestradiol production in breast cancer cells. Int J Mol Med. 2015;35:1088–94.
Brodowska K, Theodoropoulou S, Meyer ZHM, Paschalis EI, Takeuchi K, Scott G, et al. Effects of metformin on retinoblastoma growth in vitro and in vivo. Int J Oncol. 2014;45:2311–24.
Ling S, Feng T, Ke Q, Fan N, Li L, Li Z, et al. Metformin inhibits proliferation and enhances chemosensitivity of intrahepatic cholangiocarcinoma cell lines. Oncol Rep. 2014;31:2611–8.
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101.
Seront E, Pinto A, Bouzin C, Bertrand L, Machiels JP, Feron O. PTEN deficiency is associated with reduced sensitivity to mTOR inhibitor in human bladder cancer through the unhampered feedback loop driving PI3K/Akt activation. Br J Cancer. 2013;109:1586–92.
Soares HP, Ni Y, Kisfalvi K, Sinnett-Smith J, Rozengurt E. Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells. PLoS One. 2013;8, e57289.
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68.
Acknowledgments
The authors would like to thank Wu Zhang (Shanghai Institute of Hematology) for his technical assistance. This study was supported by grants from the Natural Sciences Foundation of China (no. 81172550).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xu Chen and Chuanzhen Hu contributed to this work equally as first authors.
Weibin Zhang and Yuhui Shen contributed to this work equally as corresponding authors.
Rights and permissions
About this article
Cite this article
Chen, X., Hu, C., Zhang, W. et al. Metformin inhibits the proliferation, metastasis, and cancer stem-like sphere formation in osteosarcoma MG63 cells in vitro. Tumor Biol. 36, 9873–9883 (2015). https://doi.org/10.1007/s13277-015-3751-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3751-1