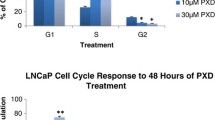
Abstract
Studies on genetic changes underlying prostate cancer and the possible signaling pathways are getting increased day by day, and new treatment methods are being searched for. The aim of the present study is to investigate the effects of ferulic acid (FA), a phenolic compound, on cell cycle, apoptosis, invasion, and colony formation in the PC-3 and LNCaP prostate cancer cells. The effect of FA on cell viability was determined via a 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) method. Total RNA was isolated with Tri Reagent. Expression of 84 genes for both cell cycle and apoptosis separately was evaluated by reverse transcriptase PCR (RT-PCR). Protein expressions were evaluated by Western blot analysis. Furthermore, apoptotic effects of FA were observed with terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) assay. Effects of FA on cell invasion and colony formation were determined using Matrigel chamber and colony assay, respectively. The half maximal inhibitory concentration (IC50) dose of FA was found to be 300 μM in PC-3 cells and 500 μM in LNCaP cells. According to RT-PCR results, it was observed that FA inhibited cell proliferation by increasing the gene expressions of ATR, ATM, CDKN1A, CDKN1B, E2F4, RB1, and TP53 and decreasing the gene expressions of CCND1, CCND2, CCND3, CDK2, CDK4, and CDK6 in PC-3 cells. On the other hand, it was seen that FA suppressed cell proliferation by increasing in the gene expressions of CASP1, CASP2, CASP8, CYCS, FAS, FASLG, and TRADD and decreasing in the gene expressions of BCL2 and XIAP in LNCaP cells. In this study, protein expression of CDK4 and BCL2 genes significantly decreased in these cells. It could induce apoptosis in PC-3 and LNCaP cells. Also, it was observed that FA suppressed the invasion in PC-3 and LNCaP cells. Moreover, it suppressed the colony formation. In conclusion, it has been observed that FA may lead to cell cycle arrest in PC-3 cells while it may cause apoptosis in LNCaP cells.





Similar content being viewed by others
Abbreviations
- FA:
-
Ferulic acid
- CDK:
-
Cyclin-dependent kinase
- DMSO:
-
Dimethyl sulfoxide
- XTT:
-
2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
- TUNEL:
-
Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling
- AR:
-
Androgen receptor
References
Szliszka E, Krol W. Polyphenols isolated from propolis augment TRAIL-induced apoptosis in cancer cells. Evid Based Complement Alternat Med. 2011. doi:10.1155/2013/731940.
Cimino S, Sortino G, Favilla V, Castelli T, Madonia M, Sansalone S, et al. Polyphenols: key issues involved in chemoprevention of prostate cancer. Oxid Med Cell Longev. 2012. doi:10.1155/2012/632959.
Chin JL, Reiter RE. Molecular markers and prostate cancer prognosis. Clin Prostate Cancer. 2004;3(3):157–64.
Velcheti V, Karnik S, Bardot SF, Prakash O. Pathogenesis of prostate cancer: lessons from basic research. Ochsner J. 2008;8(4):213–8.
Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8.
Fresco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2009;26(6):747–66.
Fresco P, Borges F, Marques MP, Diniz C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr Pharm Des. 2010;16(1):114–34.
Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. 2010;62(1):1–20.
D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita. 2007;43(4):348–61.
Clifford MN. Chlorogenic acids and other cinnamates—nature, occurrence and dietary burden. J Sci Food Agric. 1999;79:362–72.
Carbone C, Campisi A, Musumeci T, Raciti G, Bonfanti R, Puglisi G. FA-loaded lipid drug delivery systems: preparation, characterization and biological studies. Eur J Pharm Sci. 2014;52:12–20.
Janicke B, Hegardt C, Krogh M, Onning G, Akesson B, Cirenajwis HM, et al. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr Cancer. 2011;63(4):611–22.
Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273(32):20213–22.
Balk SP and Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008; doi: 10.1621/nrs.06001
Xu Y, Chen SY, Ross KN, Balk SP. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and posttranscriptional increases in cyclin D proteins. Cancer Res. 2006;66(15):7783–92.
Brooks JD, Bova GS, Isaacs WB. Allelic loss of the retinoblastoma gene in primary human prostatic adenocarcinomas. Prostate. 1995;26(1):35–9.
Ittmann MM, Wieczorek R. Alterations of the retinoblastoma gene in clinically localized, stage B prostate adenocarcinomas. Hum Pathol. 1996;27(1):28–34.
Tricoli JV, Gumerlock PH, Yao JL, Chi SG, D’Souza SA, Nestok BR, et al. Alterations of the retinoblastoma gene in human prostate adenocarcinoma. Genes Chromosomes Cancer. 1996;15(2):108–14.
Jarrard DF, Modder J, Fadden P, Fu V, Sebree L, Heisey D, et al. Alterations in the p16/pRb cell cycle checkpoint occur commonly in primary and metastatic human prostate cancer. Cancer Lett. 2002;185(2):191–9.
Peng CC, Chyau CC, Wang HE, Chang CH, Chen KC, Chou KY, et al. Cytotoxicity of Ferulic acid on T24 cell line differentiated by different microenvironments. BioMed Res Int. 2013. doi:10.1155/2013/579859.
Hemaiswarya S, Doble M. Combination of phenylpropanoids with 5-fluorouracil as anti-cancer agents against human cervical cancer (HeLa) cell line. Phytomedicine. 2013;20(2):151–8.
Cho SD, Li G, Hu H, Jiang C, Kang KS, Lee YS, et al. Involvement of c-Jun N-terminal kinase in G2/M arrest and caspase-mediated apoptosis induced by sulforaphane in DU145 prostate cancer cells. Nutr Cancer. 2005;52(2):213–24.
Karanika S, Karantanos T, Li L, Corn PG, Thompson TC. DNA damage response and prostate cancer: defects, regulation and therapeutic implications. Oncogene. 2014. doi:10.1038/onc.2014.238.
Hou Y, Yang J, Zhao G, Yuan Y. Ferulic acid inhibits endothelial cell proliferation through NO downregulating ERK1/2 pathway. J Cell Biochem. 2004;93(6):1203–9.
Dong Q, Meng P, Wang T, Qin W, Wang F, Yuan J, et al. MicroRNA let-7a inhibits proliferation of human prostate cancer cells in vitro and in vivo by targeting E2F2 and CCND2. PLoS One. 2010;5(4):e10147.
Susaki E, Nakayama K, Nakayama KI. Cyclin D2 translocates p27 out of the nucleus and promotes its degradation at the G0-G1 transition. Mol Cell Biol. 2007;27(13):4626–40.
Nikoleishvili D, Pertia A, Trsintsadze O, Gogokhia N, Managadze L, Chkhotua A. Expression of p27(Kip1), cyclin D3 and Ki67 in BPH, prostate cancer and hormone-treated prostate cancer cells. Int Urol Nephrol. 2008;40(4):953–9.
D’Angiolella V, Donato V, Vijayakumar S, Saraf A, Florens L, Washburn MP, et al. SCF(cyclin F) controls centrosome homeostasis and mitotic fidelity via CP110 degradation. Nature. 2010;466(7302):138–42.
Zieske JD. Expression of cyclin-dependent kinase inhibitors during corneal wound repair. Prog Retin Eye Res. 2000;19(3):257–70.
Sirma H, Broemel M, Stumm L, Tsourlakis T, Steurer S, Tennstedt P, et al. Loss of CDKN1B/p27Kip1 expression is associated with ERG fusion-negative prostate cancer, but is unrelated to patient prognosis. Oncol Lett. 2013;6(5):1245–52.
Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the Rb protein. Cell. 1991;65(6):1053–62.
Plesca D, Crosby ME, Gupta D, Almasan A. E2F4 function in G2: maintaining G2-arrest to prevent mitotic entry with damaged DNA. Cell Cycle. 2007;6(10):1147–52.
Nagata S. Apoptosis by death factor. Cell. 1997;88(3):355–65.
Chinnadurai G, Vijayalingam S, Rashmi R. BIK, the founding member of the BH3-only family proteins: mechanisms of cell death and role in cancer and pathogenic processes. Oncogene. 2008;27(1):20–9.
Bandagula VR, Prasad NR. 2-Deoxy-glucose and ferulic acid modulates radiation response signaling in non-small cell lung cancer cells. Tumour Biol. 2013;34(1):251–9.
Guo Y, Kyprianou N. Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res. 1999;59(6):1366–71.
Winter RN, Kramer A, Borkowski A, Kyprianou N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001;61(3):1227–32.
VanOosten RL, Earel Jr JK, Griffith TS. Histone deacetylase inhibitors enhance Ad5-TRAIL killing of TRAIL-resistant prostate tumor cells through increased caspase-2 activity. Apoptosis. 2007;12(3):561–71.
Kampa M, Alexaki VI, Notas G, Nifli AP, Nistikaki A, Hatzoglou A, et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res. 2004;6(2):63–74.
Krajewska M, Krajewski S, Banares S, Huang X, Turner B, Bubendorf L, et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003;9(13):4914–25.
Ramp U, Krieg T, Caliskan E, Mahotka C, Ebert T, Willers R, et al. XIAP expression is an independent prognostic marker in clear-cell renal carcinomas. Hum Pathol. 2004;35(8):1022–8.
Karthikeyan S, Kanimozhi G, Prasad NR, Mahalakshmi R. Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol In Vitro. 2011;25(7):1366–75.
Tsai CM, Yen GC, Sun FM, Yang SF, Weng CJ. Assessment of the anti-invasion potential and mechanism of select cinnamic acid derivatives on human lung adenocarcinoma cells. Mol Pharm. 2013;10(5):1890–900.
Fang HY, Wang HM, Chang KF, Hu HT, Hwang LJ, Fu TF, et al. Feruloyl-L-arabinose attenuates migration, invasion and production of reactive oxygen species in H1299 lung cancer cells. Food ChemToxicol. 2013;58:459–66. doi:10.1016/j.fct.2013.05.019.
Acknowledgments
This study was produced from the MSc thesis of Canan Eroglu and supported by Instructor Training Program.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eroğlu, C., Seçme, M., Bağcı, G. et al. Assessment of the anticancer mechanism of ferulic acid via cell cycle and apoptotic pathways in human prostate cancer cell lines. Tumor Biol. 36, 9437–9446 (2015). https://doi.org/10.1007/s13277-015-3689-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3689-3