Abstract
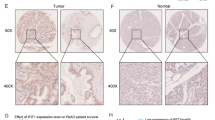
Downregulated parafibromin expression is involved in the pathogenesis and progression of parathyroid, breast, gastric, colorectal, and lung cancers. To investigate the roles of parafibromin expression in tumorigenesis, progression, and prognostic evaluation of head and neck squamous cell carcinomas (HNSCCs), we transfected parafibromin-expressing plasmid into HNSCC cell and examined the phenotypes and their relevant molecules. Parafibromin expression was detected on tissue microarray containing squamous epithelium, dysplasia, and carcinoma of head and neck by immunohistochemistry. Parafibromin overexpression was found to suppress growth, migration, and invasion, and induce apoptosis, S arrest, and mesenchymal to epithelial transition (EMT), compared with the mock and control (P < 0.05). Both overexpression of Cyclin E1, Bax, and E-cadherin and hypoexpression of c-myc, Bcl-xL, and slug were detected in B88 transfectants, in comparison to mock and control by real-time PCR. Parafibromin expression was weaker in primary cancers than those in normal squamous tissue and dysplasia (P < 0.05), but stronger than the metastatic cancers in lymph node (P < 0.05). Parafibromin expression was negatively correlated with lymph node metastasis, tumor-node-metastasis (TNM) staging, but positively with human papillomavirus (HPV) positivity (P < 0.05). The HNSCCs in tongue showed more parafibromin expression than those in larynx (P < 0.05). There was stronger parafibromin expression in moderately-than poorly-differentiated carcinomas (P < 0.05). The significantly positive correlation was observed between parafibromin expression and relapse-free survival rate by Kaplan-Meier curves (P < 0.05). Cox’s proportional hazard model indicated that distant metastasis and parafibromin expression were independent prognostic factors for overall and relapse-free survival of HNSCC, respectively (P < 0.05). These findings suggest that downregulated expression of parafibromin protein plays an important role in the pathogenesis, differentiation, and metastasis of HNSCCs possibly by inducing apoptosis, suppressing proliferation, cell cycle progression, migration, invasion, and EMT. Parafibromin expression is an independent factor for relapse-free survival of HNSCCs.




Similar content being viewed by others
References
Agarwal SK, Simonds WF, Marx SJ. The parafibromin tumor suppressor protein interacts with actin-binding proteins actinin-2 and actinin-3. Mol Cancer. 2008;7:65.
Aldred MJ, Talacko AA, Savarirayan R, Murdolo V, Mills AE, Radden BG, et al. Dental findings in a family with hyperparathyroidism-jaw tumour syndrome and a novel HRPT2 gene mutation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:212–8.
Barnes L, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization classification of tumors. Pathology and head and neck tumors. Lyon: IARC. 2005; 9-208.
Bradley KJ, Bowl MR, Williams SE, Ahmad BN, Partridge CJ, Patmanidi AL, et al. Parafibromin is a nuclear protein with a functional monopartite nuclear localization signal. Oncogene. 2007;26:1213–21.
Cetani F, Banti C, Pardi E, Borsari S, Viacava P, Miccoli P, et al. CDC73 mutational status and loss of parafibromin in the outcome of parathyroid cancer. Endocr Connect. 2013;2:186–95.
Cooper T, Biron VL, Fast D, Tam R, Carey T, Shmulevitz M, et al. Oncolytic activity of reovirus in HPV positive and negative head and neck squamous cell carcinoma. J Otolaryngol Head Neck Surg. 2015;44:8.
Cosentino K, García-Sáez AJ. Mitochondrial alterations in apoptosis. Chem Phys Lipids. 2014;181:62–75.
Gollin SM. Cytogenetic alterations and their molecular genetic correlates in head and neck squamous cell carcinoma: a next generation window to the biology of disease. Genes Chromosomes Cancer. 2014;53:972–90.
Gou WF, Zhao Y, Lu H, Yang XF, Xiu YL, Zhao S, et al. The role of RhoC in epithelial-to-mesenchymal transition of ovarian carcinoma cells. BMC Cancer. 2014;14:477.
Hahn MA, Dickson KA, Jackson S, Clarkson A, Gill AJ, Marsh DJ. The tumor suppressor CDC73 interacts with the ring finger proteins RNF20 and RNF40 and is required for the maintenance of histone 2B monoubiquitination. Hum Mol Genet. 2012;21:559–68.
Iwata T, Mizusawa N, Taketani Y, Itakura M, Yoshimoto K. Parafibromin tumor suppressor enhances cell growth in the cells expressing SV40 large T antigen. Oncogene. 2007;26:6176–83.
Jo JH, Chung TM, Youn H, Yoo JY. Cytoplasmic parafibromin/hCdc73 targets and destabilizes p53 mRNA to control p53-mediated apoptosis. Nat Commun. 2014;5:5433.
Kim L, King T, Agulnik M. Head and neck cancer: changing epidemiology and public health implications. Oncology (Williston Park). 2010;24:915–9.
Kumada T, Tsuneyama K, Hatta H, Ishizawa S, Takano Y. Improved 1-h rapid immunostaining method using intermittent microwave irradiation: practicability based on 5 years application in Toyama Medical and Pharmaceutical University Hospital. Mod Pathol. 2004;17:1141–9.
Lin L, Czapiga M, Nini L, Zhang JH, Simonds WF. Nuclear localization of the parafibromin tumor suppressor protein implicated in the hyperparathyroidism-jaw tumor syndrome enhances its proapoptotic function. Mol Cancer Res. 2007;5:183–93.
Lin L, Zhang JH, Panicker LM, Simonds WF. The parafibromin tumor suppressor protein inhibits cell proliferation by repression of the c-myc proto-oncogene. Proc Natl Acad Sci U S A. 2008;105:17420–5.
Masi G, Iacobone M, Sinigaglia A, Mantelli B, Pennelli G, Castagliuolo I, et al. Characterization of a new CDC73 missense mutation that impairs parafibromin expression and nucleolar localization. PLoS One. 2014;9, e97994.
Rather MI, Swamy S, Gopinath KS, Kumar A. Transcriptional repression of tumor suppressor CDC73, encoding an RNA polymerase II interactor, by Wilms tumor 1 protein (WT1) promotes cell proliferation: implication for cancer therapeutics. J Biol Chem. 2014;289:968–76.
Rozenblatt-Rosen O, Hughes CM, Nannepaga SJ, Shanmugam KS, Copeland TD, Guszczynski T, et al. The parafibromin tumour suppressor protein is part of a human Paf1 complex. Mol Cell Biol. 2005;25:612–20.
Selvarajan S, Sii LH, Lee A, Yip G, Bay BH, Tan MH, et al. Parafibromin expression in breast cancer: a novel marker for prognostication? J Clin Pathol. 2008;61:64–7.
Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D, et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349:1722–9.
Sobin LH. TNM classification of malignant tumors. 7th ed. Hoboken: Wiley; 2009.
Tan MH, Wong CF, Tan HL, Yang XJ, Ditlev J, Matsuda D, et al. Genomic expression and single-nucleotide polymorphism profiling discriminates chromophobe renal cell carcinoma and oncocytoma. BMC Cancer. 2010;10:196.
Williams G, Stoeber K. The cell cycle and cancer. J Pathol. 2012;226:352–64.
Woodard GE, Lin L, Zhang JH, Agarwal SK, Marx SJ, Simonds WF. Parafibromin, product of the hyperparathyroidism-jaw tumor syndrome gene HRPT2, regulates cyclin D1/PRAD1 expression. Oncogene. 2005;24:1272–6.
Xia P, Wang W, Xu XY, Wang JP, Takano Y, Zheng HC. Parafibromin expression in lung normal tissue and carcinoma: its comparison with clinicopathological parameters of carcinoma. Histol Histopathol. 2011;26:1039–47.
Yang YJ, Han JW, Youn HD, Cho EJ. The tumor suppressor, parafibromin, mediates histone H3 K9 methylation for cyclin D1 repression. Nucleic Acids Res. 2010;38:382–90.
Yart A, Gstaiger M, Wirbelauer C, Pecnik M, Anastasiou D, Hess D, et al. The HRPT2 tumour suppressor gene product parafibromin associates with human PAF1 and RNA polymerase II. Mol Cell Biol. 2007;25:5052–60.
Zhao S, Sun HZ, Zhu ST, Lu H, Niu ZF, Guo WF, et al. Effects of Parafibromin expression on the phenotypes and relevant mechanisms in the DLD-1 colon carcinoma cell line. Asian Pac J Cancer Prev. 2013;14:4249–54.
Zheng HC, Nakamura T, Zheng Y, Nakanishi Y, Tabuchi Y, Uchiyama A, et al. SV40 T antigen disrupted the cell metabolism and the balance between proliferation and apoptosis in lens tumors of transgenic mice. J Cancer Res Clin Oncol. 2009;135:1521–32.
Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, et al. Downregulated parafibromin expression is a promising marker for pathogenesis, invasion, metastasis and prognosis of gastric carcinomas. Virchows Arch. 2008;452:147–55.
Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579–83.
Zheng HC, Wei ZL, Xu XY, Nie XC, Yang X, Takahashi H, et al. Parafibromin expression is an independent prognostic factor for colorectal carcinomas. Hum Pathol. 2011;42:1089–102.
Zheng Y, Xia P, Zheng HC, Takahashi H, Masuda S, Takano Y. The screening of viral risk factors in tongue and pharyngolaryngeal squamous carcinoma. Anticancer Res. 2010;30:1233–8.
Acknowledgments
This study was supported by President Fund of Liaoning Medical University (XZJJ20140201; XZJJ20140203), a Project Supported by Scientific Research Fund of Liaoning Provincial Education Department (LJQ2014093) and National Natural Scientific Foundation of China (81172371; 81472544).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z., Yang, Xf., Huang, Kq. et al. The clinicopathological significances and biological functions of parafibromin expression in head and neck squamous cell carcinomas. Tumor Biol. 36, 9487–9497 (2015). https://doi.org/10.1007/s13277-015-3618-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3618-5