Abstract
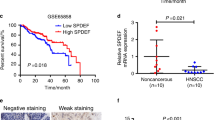
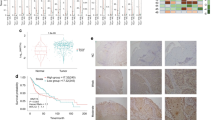
Expression of the protein deacetylase SIRT1 is associated with either poor or favorable prognosis in cancer patients, depending on the cancer type. In head and neck squamous cell carcinoma (HNSCC), SIRT1 expression is associated with favorable prognosis. However, the molecular mechanism underlying the tumor-suppressive function of SIRT1 in HNSCC is unknown. SIRT1 promotes differentiation in epithelial cells; therefore, we investigated whether SIRT1 promotes differentiation in HNSCC cells by studying the correlations between the expression of SIRT1 and several genes implicated in stemness or differentiation in HNSCC-derived cell lines. Our results suggest that SIRT1 does not contribute to differentiation in HNSCC cells. RNA interference-mediated reduction of SIRT1 revealed that SIRT1 supports the expression of TAp63, which has been implicated in tumor suppression, in addition to epithelial differentiation. A positive correlation was observed between SIRT1 and TAp63 expression in HNSCC tissues, as determined by quantitative reverse transcription-polymerase chain reaction analysis of RNA extracted from formalin-fixed paraffin-embedded biopsy samples. Together, these results suggest that although SIRT1 does not regulate differentiation of HNSCC cells, it functions as a tumor suppressor in HNSCC by supporting the transcription of tumor-suppressive TAp63. This finding supports the notion that SIRT1-activating drugs could be useful for the treatment of HNSCC.





Similar content being viewed by others
References
Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–35. doi:10.1146/annurev.biochem.73.011303.073651.
Zhang T, Kraus WL. SIRT1-dependent regulation of chromatin and transcription: linking NAD(+) metabolism and signaling to the control of cellular functions. Biochim Biophys Acta. 2010;1804(8):1666–75. doi:10.1016/j.bbapap.2009.10.022.
Yuan H, Su L, Chen WY. The emerging and diverse roles of sirtuins in cancer: a clinical perspective. Onco Targets Ther. 2013;6:1399–416. doi:10.2147/OTT.S37750.
Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–95. doi:10.1146/annurev.pathol.4.110807.092250.
Sebastian C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012;287(51):42444–52. doi:10.1074/jbc.R112.402768.
Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010;10(12):819–23. doi:10.1038/nrc2962.
Villalba JM, Alcain FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38(5):349–59. doi:10.1002/biof.1032.
Roth M, Chen WY. Sorting out functions of sirtuins in cancer. Oncogene. 2014;33(13):1609–20. doi:10.1038/onc.2013.120.
Noguchi A, Li X, Kubota A, Kikuchi K, Kameda Y, Zheng H, et al. SIRT1 expression is associated with good prognosis for head and neck squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(3):385–92. doi:10.1016/j.oooo.2012.12.013.
Lai CC, Lin PM, Lin SF, Hsu CH, Lin HC, Hu ML, et al. Altered expression of SIRT gene family in head and neck squamous cell carcinoma. Tumour Biol. 2013;34(3):1847–54. doi:10.1007/s13277-013-0726-y.
Chen IC, Chiang WF, Huang HH, Chen PF, Shen YY, Chiang HC. Role of SIRT1 in regulation of epithelial-to-mesenchymal transition in oral squamous cell carcinoma metastasis. Mol Cancer. 2014;13:254. doi:10.1186/1476-4598-13-254.
Jang KY, Kim KS, Hwang SH, Kwon KS, Kim KR, Park HS, et al. Expression and prognostic significance of SIRT1 in ovarian epithelial tumours. Pathology. 2009;41(4):366–71. doi:10.1080/00313020902884451.
Cha EJ, Noh SJ, Kwon KS, Kim CY, Park BH, Park HS, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res. 2009;15:4453–9. doi:10.1158/1078-0432.CCR-08-3329.
Lee H, Kim KR, Noh SJ, Park HS, Kwon KS, Park BH, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis for breast carcinoma. Hum Pathol. 2011;42:204–13. doi:10.1016/j.humpath.2010.05.023.
Chen HC, Jeng YM, Yuan RH, Hsu HC, Chen YL. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol. 2012;19(6):2011–9. doi:10.1245/s10434-011-2159-4.
Noguchi A, Kikuchi K, Zheng H, Takahashi H, Miyagi Y, Aoki I, et al. SIRT1 expression is associated with a poor prognosis, whereas DBC1 is associated with favorable outcomes in gastric cancer. Cancer Med. 2014;3(6):1553–61. doi:10.1002/cam4.310.
Nosho K, Shima K, Irahara N, Kure S, Firestein R, Baba Y, et al. SIRT1 histone deacetylase expression is associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Mod Pathol. 2009;22:922–32. doi:10.1038/modpathol.2009.49.
Jang SH, Min KW, Paik SS, Jang KS. Loss of SIRT1 histone deacetylase expression associates with tumour progression in colorectal adenocarcinoma. J Clin Pathol. 2012;65(8):735–9. doi:10.1136/jclinpath-2012-200685.
Kikuchi K, Noguchi A, Takahashi H, Zheng H, Kameda Y, Sekiguchi H, et al. High SIRT1 expression and low DBC1 expression are associated with poor prognosis in colorectal cancer. J Cancer Therapeut Res. 2013;2:1–8. doi:10.7243/2050-120X-2-1.
Jung W, Hong KD, Jung WY, Lee E, Shin BK, Kim HK, et al. SIRT1 expression is associated with good prognosis in colorectal cancer. Korean J Pathol. 2013;47(4):332–9. doi:10.4132/KoreanJPathol.2013.47.4.332.
Lv L, Shen Z, Zhang J, Zhang H, Dong J, Yan Y, et al. Clinicopathological significance of SIRT1 expression in colorectal adenocarcinoma. Med Oncol. 2014;31(6):965. doi:10.1007/s12032-014-0965-9.
Milner J. Cellular regulation of SIRT1. Curr Pharmaceut Des. 2009;15:39–44. doi:10.2174/138161209787185841.
Deng C. SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci. 2009;5:147–52. doi:10.7150/ijbs.5.147.
Bosch-Presegué L, Vaquero A. The dual role of sirtuins in cancer. Genes Cancer. 2011;2:648–62. doi:10.1177/1947601911417862.
Blander G, Bhimavarapu A, Mammone T, Maes D, Elliston K, Reich C, et al. SIRT1 promotes differentiation of normal human keratinocytes. J Invest Dermatol. 2009;129(1):41–9. doi:10.1038/jid.2008.179.
Yuan J, Minter-Dykhouse K, Lou Z. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol. 2009;185(2):203–11. doi:10.1083/jcb.200809167.
Mao B, Zhao G, Lv X, Chen HZ, Xue Z, Yang B, et al. Sirt1 deacetylates c-Myc and promotes c-Myc/Max association. Int J Biochem Cell Biol. 2011;43(11):1573–81. doi:10.1016/j.biocel.2011.07.006.
Menssen A, Hydbring P, Kapelle K, Vervoorts J, Diebold J, Luscher B, et al. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc Natl Acad Sci U S A. 2012;109(4):E187–196. doi:10.1073/pnas.1105304109.
Watt FM, Frye M, Benitah SA. MYC in mammalian epidermis: how can an oncogene stimulate differentiation? Nat Rev Cancer. 2008;8(3):234–42. doi:10.1038/nrc2328.
Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143(2):313–24. doi:10.1016/j.cell.2010.09.010.
Evers DL, He J, Kim YH, Mason JT, O’Leary TJ. Paraffin embedding contributes to RNA aggregation, reduced RNA yield, and low RNA quality. J Mol Diagn. 2011;13(6):687–94. doi:10.1016/j.jmoldx.2011.06.007.
Candi E, Dinsdale D, Rufini A, Salomoni P, Knight RA, Mueller M, et al. TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle. 2007;6(3):274–85. doi:10.4161/cc.6.3.3797.
Mulder KW, Wang X, Escriu C, Ito Y, Schwarz RF, Gillis J, et al. Diverse epigenetic strategies interact to control epidermal differentiation. Nat Cell Biol. 2012;14(7):753–63. doi:10.1038/ncb2520.
Tan DW, Jensen KB, Trotter MW, Connelly JT, Broad S, Watt FM. Single-cell gene expression profiling reveals functional heterogeneity of undifferentiated human epidermal cells. Development. 2013;140(7):1433–44. doi:10.1242/dev.087551.
Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B. Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun. 2009;383(2):157–62. doi:10.1016/j.bbrc.2009.02.156.
Lim YC, Oh SY, Cha YY, Kim SH, Jin X, Kim H. Cancer stem cell traits in squamospheres derived from primary head and neck squamous cell carcinomas. Oral Oncol. 2011;47(2):83–91. doi:10.1016/j.oraloncology.2010.11.011.
Chiou S, Yu C, Huang C, Lin S, Liu S, Tsai T, et al. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2013;14:4085–95. doi.
Dallaglio K, Petrachi T, Marconi A, Truzzi F, Lotti R, Saltari A, et al. Isolation and characterization of squamous cell carcinoma-derived stem-like cells: role in tumor formation. Int J Mol Sci. 2013;14(10):19540–55. doi:10.3390/ijms141019540.
Melino G. p63 is a suppressor of tumorigenesis and metastasis interacting with mutant p53. Cell Death Differ. 2011;18(9):1487–99. doi:10.1038/cdd.2011.81.
Yoshikawa M, Tsuchihashi K, Ishimoto T, Yae T, Motohara T, Sugihara E, et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1855–66. doi:10.1158/0008-5472.CAN-12-3609-T.
Kikuchi K, Tsutsumi K, Ohta Y, Yasumoto S. Time correlation of commitment to calcium-induced apoptosis and terminal differentiation in human ectocervical keratinocytes in suspension cultures. Cell Growth Differ. 1997;8(5):571–9.
Matsubara R, Kawano S, Kiyosue T, Goto Y, Hirano M, Jinno T, et al. Increased DeltaNp63 expression is predictive of malignant transformation in oral epithelial dysplasia and poor prognosis in oral squamous cell carcinoma. Int J Oncol. 2011;39(6):1391–9. doi:10.3892/ijo.2011.1151.
Yang X, Lu H, Yan B, Romano RA, Bian Y, Friedman J, et al. DeltaNp63 versatilely regulates a Broad NF-kappaB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res. 2011;71(10):3688–700. doi:10.1158/0008-5472.CAN-10-3445.
Chakrabarti R, Wei Y, Hwang J, Hang X, Andres Blanco M, Choudhury A, et al. DeltaNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat Cell Biol. 2014;16(10):1004–15. doi:10.1038/ncb3040.
Sommer M, Poliak N, Upadhyay S, Ratovitski E, Nelkin BD, Donehower LA, et al. DeltaNp63alpha overexpression induces downregulation of Sirt1 and an accelerated aging phenotype in the mouse. Cell Cycle. 2006;5(17):2005–11. doi.
Guo X, Keyes WM, Papazoglu C, Zuber J, Li W, Lowe SW, et al. TAp63 induces senescence and suppresses tumorigenesis in vivo. Nat Cell Biol. 2009;11(12):1451–7. doi:10.1038/ncb1988.
Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin YL, et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature. 2010;467(7318):986–90. doi:10.1038/nature09459.
Mattiske S, Ho K, Noll JE, Neilsen PM, Callen DF, Suetani RJ. TAp63 regulates oncogenic miR-155 to mediate migration and tumour growth. Oncotarget. 2013;4(11):1894–903. doi.
Tan EH, Morton JP, Timpson P, Tucci P, Melino G, Flores ER, et al. Functions of TAp63 and p53 in restraining the development of metastatic cancer. Oncogene. 2014;33(25):3325–33. doi:10.1038/onc.2013.287.
Su X, Gi YJ, Chakravarti D, Chan IL, Zhang A, Xia X, et al. TAp63 is a master transcriptional regulator of lipid and glucose metabolism. Cell Metab. 2012;16(4):511–25. doi:10.1016/j.cmet.2012.09.006.
Acknowledgments
The authors would like to thank Mitsuyo Yoshihara (Kanagawa Cancer Center Research Institute) for the assistance in RNA extraction from FFPE samples. This study was partly supported by a Grant-in-Aid for Scientific Research to KK (24593034) from the Ministry of Education, Science, Sports, and Culture.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Semi-quantitative analysis of the effect of SIRT1 reduction on the expression of genes involved in the differentiation and maintenance of stemness in KOSC2 cells. Right panels show densitometry results of western blot analysis of SIRT1 or RT-PCR products normalized relative to G3PDH as 1. (JPEG 459 kb)
Supplementary Table 1
Quantification of gene expression in HNSCC cell lines shown in Figure 1. (DOCX 74 kb)
Supplementary Table 2
Clinicopathological parameters of 20 HNSCC biopsy samples analyzed in this study. (DOCX 72 kb)
Rights and permissions
About this article
Cite this article
Kikuchi, K., Noguchi, A., Kasajima, R. et al. Association of SIRT1 and tumor suppressor gene TAp63 expression in head and neck squamous cell carcinoma. Tumor Biol. 36, 7865–7872 (2015). https://doi.org/10.1007/s13277-015-3515-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3515-y