Abstract
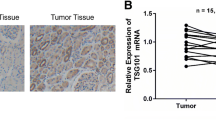
Nuclear auto-antigenic sperm protein (NASP), initially described as a highly auto-immunogenic testis and sperm-specific protein, is a histone chaperone that is proved to present in all dividing cells. NASP has two splice variants: testicular NASP (tNASP) and somatic form of NASP (sNASP). Only cancer, germ, transformed, and embryonic cells have a high level of expression of the tNASP. Up to now, little has been known about tNASP in renal cell carcinoma (RCC). In the present study, the molecular mechanism of tNASP in RCC was explored. The expression level of tNASP in 16 paired human RCC specimens was determined. Downregulation of tNASP by small interfering RNA (siRNA) was transfected in RCC cell lines. The effect of downregulation of tNASP by siRNA on cell colony formation and proliferation was examined by colony formation assay and CCK-8 assay, cell cycle was analyzed by flow cytometry, and the expression of cyclin D1 and P21 were detected by Western blotting. ERK/MAPK signaling was also analyzed. tNASP has a relative high expression level in human RCC tissues. Via upregulation of P21 and downregulation of cyclinD1, silence of tNASP can inhibit cell proliferation, which induces cell cycle arrest. Furthermore, ERK signaling pathway is confirmed to mediate the regulation of cell cycle-related proteins caused by silence of tNASP. Our research demonstrates that knockdown of tNASP effectively inhibits the proliferation and causes G1 phase arrest through ERK/MAPK signal pathway.




Similar content being viewed by others
Reference
Chow WH, Devesa SS, Warren JL, Fraumeni Jr JF. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281(17):1628–31.
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34(3):193–205.
Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007;109(9):1763–8.
Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373(9669):1119–32.
Jiang Z, Chu PG, Woda BA, Liu Q, Balaji KC, et al. Combination of quantitative IMP3 and tumor stage: a new system to predict metastasis for patients with localized renal cell carcinomas. Clin Cancer Res. 2008;14(17):5579–84.
Finn RM, Browne K, Hodgson KC, Ausió J. sNASP, a histone H1-specific eukaryotic chaperone dimer that facilitates chromatin assembly. Biophys J. 2008;95(3):1314–25.
Richardson RT, Batova IN, Widgren EE, Zheng LX, Whitfield M, et al. Characterization of the histone H1-binding protein, NASP, as a cell cycle-regulated somatic protein. J Biol Chem. 2000;275(39):30378–86.
Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116(1):51–61.
Wang H, Walsh ST, Parthun MR. Expanded binding specificity of the human histone chaperone NASP. Nucleic Acids Res. 2008;36(18):5763–72.
Alekseev OM, Richardson RT, Alekseev O, O'Rand MG. Analysis of gene expression profiles in HeLa cells in response to overexpression or siRNA-mediated depletion of NASP. Reprod Biol Endocrinol. 2009;7:45.
Richardson RT, Alekseev OM, Grossman G, Widgren EE, Thresher R, et al. Nuclear autoantigenic sperm protein (NASP), a linker histone chaperone that is required for cell proliferation. J Biol Chem. 2006;281(30):21526–34.
Chatterjee M, Mohapatra S, Ionan A, Bawa G, Ali-Fehmi R, et al. Diagnostic markers of ovarian cancer by high-throughput antigen cloning and detection on arrays. Cancer Res. 2006;66(2):1181–90.
Amundson SA, Do KT, Vinikoor LC, Lee RA, Koch-Paiz CA, et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res. 2008;68(2):415–24.
Martin KJ, Patrick DR, Bissell MJ, Fournier MV. Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS One. 2008;3(8):e2994.
van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–6.
Alekseev OM, Richardson RT, Tsuruta JK, O'Rand MG. Depletion of the histone chaperone tNASP inhibits proliferation and induces apoptosis in prostate cancer PC-3 cells. Reprod Biol Endocrinol. 2011;9(1):50.
Tashiro E, Tsuchiya A, Imoto M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Cancer Sci. 2007;98(5):629–35.
Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–66.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87.
Lam JS, Breda A, Belldegrun AS, Figlin RA. Evolving principles of surgical management and prognostic factors for outcome in renal cell carcinoma. J Clin Oncol. 2006;24(35):5565–75.
Rini BI. Metastatic renal cell carcinoma: many treatment options, one patient. J Clin Oncol. 2009;27(19):3225–34.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81270685).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianzheng Fang, Hainan Wang and Wei Xi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fang, J., Wang, H., Xi, W. et al. Downregulation of tNASP inhibits proliferation through regulating cell cycle-related proteins and inactive ERK/MAPK signal pathway in renal cell carcinoma cells. Tumor Biol. 36, 5209–5214 (2015). https://doi.org/10.1007/s13277-015-3177-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3177-9