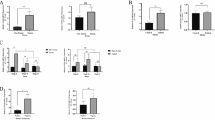
Abstract
The CD95 pathway is a critical apoptotic pathway used by immune cells to avoid cancer development. CD95 ligand (CD95L) is found in several forms, as a cell membrane-associated form, a soluble metalloprotease-cleaved form, and a soluble but membrane-bound CD95L released on cell-derived exosomes. In this study, we used a cell-based assay to evaluate the activity of proapoptotic CD95L in sera from healthy individuals and breast cancer patients. We confirmed that our cell-based assay using Jurkat cells was sensitive to the presence of proapoptotic CD95L in serum, and apoptosis induction by mechanisms other than CD95 was discriminated using apoptosis-resistant Jurkat subclones. Our results indicated a proapoptotic potential of normal serum that involved CD95L. Sera from breast cancer patients exhibited significantly decreased apoptosis induction, due to increased CD95 receptor levels compared with healthy women. Apoptotic potential tended to decrease as the Breast Imaging Reporting and Data System grade increased, and we observed restoration of proapoptotic potential after tumor removal. The CD95L in serum responsible for apoptotic induction was associated with high-molecular-weight particles, perhaps with exosomes. The sera of healthy individuals generally contain a proapoptotic environment, and this property is mainly maintained by the presence of CD95L. Furthermore, measurement of CD95L-mediated apoptosis induction by sera could be a useful parameter to be evaluated during cancer development and therapeutic response.






Similar content being viewed by others
References
Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–6. doi:10.1038/35037710.
Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohme I, Forsberg B, et al. Cancer risk after renal transplantation in the Nordic countries, 1964-1986. Int J Cancer. 1995;60(2):183–9.
Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23):2823–31.
Bonnet F, Chene G. Evolving epidemiology of malignancies in HIV. Curr Opin Oncol. 2008;20(5):534–40. doi:10.1097/CCO.0b013e32830a5080.
Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118(Pt 2):265–7. doi:10.1242/jcs.01610.
Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C, et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Investig. 1993;69(4):415–29.
Keane MM, Ettenberg SA, Lowrey GA, Russell EK, Lipkowitz S. Fas expression and function in normal and malignant breast cell lines. Cancer Res. 1996;56(20):4791–8.
Gupta S, Su H, Bi R, Agrawal S, Gollapudi S. Life and death of lymphocytes: a role in immunesenescence. Immun Ageing. 2005;2:12. doi:10.1186/1742-4933-2-12.
Martinez-Lorenzo MJ, Anel A, Gamen S, Monle NI, Lasierra P, Larrad L. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J Immunol. 1999;163(3):1274–81.
Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M, et al. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167(12):6736–44.
Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res: Off J Am Assoc Cancer Res. 2005;11(3):1010–20.
Penna A, Khadra N, Tauzin S, Vacher P, Legembre P. The CD95 signaling pathway: to not die and fly. Commun Integr Biol. 2012;5(2):190–2. doi:10.4161/cib.18888.
Lu L, Qian S, Hershberger PA, Rudert WA, Lynch DH, Thomson AW. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol. 1997;158(12):5676–84.
Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, et al. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol. 1998;19(4):537–42. doi:10.1165/ajrcmb.19.4.3100.
Walczak H. Death receptor-ligand systems in cancer, cell death, and inflammation. Cold Spring Harbor Perspect Biol. 2013;5(5):a008698. doi:10.1101/cshperspect.a008698.
Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, et al. Fas ligand in human serum. Nat Med. 1996;2(3):317–22.
Gillis S, Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980;152(6):1709–19.
Weis M, Schlegel J, Kass GE, Holmstrom TH, Peters I, Eriksson J, et al. Cellular events in Fas/APO-1-mediated apoptosis in JURKAT T lymphocytes. Exp Cell Res. 1995;219(2):699–708. doi:10.1006/excr.1995.1281.
Martinez-Lorenzo MJ, Alava MA, Anel A, Pineiro A, Naval J. Release of preformed Fas ligand in soluble form is the major factor for activation-induced death of Jurkat T cells. Immunology. 1996;89(4):511–7.
Peter ME, Dhein J, Ehret A, Hellbardt S, Walczak H, Moldenhauer G, et al. APO-1 (CD95)-dependent and -independent antigen receptor-induced apoptosis in human T and B cell lines. Int Immunol. 1995;7(11):1873–7.
Juo P, Woo MS, Kuo CJ, Signorelli P, Biemann HP, Hannun YA, et al. FADD is required for multiple signaling events downstream of the receptor Fas. Cell Growth Diff: Molec Biol J Am Assoc Cancer Res. 1999;10(12):797–804.
Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol: CB. 1998;8(18):1001–8.
Sedgwick E. The breast ultrasound lexicon: breast imaging reporting and data system (BI-RADS). Semin Roentgenol. 2011;46(4):245–51. doi:10.1053/j.ro.2011.04.001.
Sheen-Chen SM, Chen HS, Eng HL, Chen WJ. Circulating soluble Fas in patients with breast cancer. World J Surg. 2003;27(1):10–3. doi:10.1007/s00268-002-6378-5.
Hewala TI, Abd El-Monaim NA, Anwar M, Ebied SA. The clinical significance of serum soluble Fas and p53 protein in breast cancer patients: comparison with serum CA 15-3. Pathol Oncol Res. 2012;18(4):841–8. doi:10.1007/s12253-012-9512-1.
Kato K, Ohshima K, Ishihara S, Anzai K, Suzumiya J, Kikuchi M. Elevated serum soluble Fas ligand in natural killer cell proliferative disorders. Br J Haematol. 1998;103(4):1164–6.
Mizutani Y, Hongo F, Sato N, Ogawa O, Yoshida O, Miki T. Significance of serum soluble Fas ligand in patients with bladder carcinoma. Cancer. 2001;92(2):28–93.
Mizutani Y, Hongo F, Sato N, Ogawa O, Yoshida O, Miki T. Significance of serum soluble Fas ligand in patients with bladder carcinoma. Cancer. 2001;45(3):199–204.
Malleter M, Tauzin S, Bessede A, Castellano R, Goubard A, Godey F, et al. CD95L cell surface cleavage triggers a prometastatic signaling pathway in triple-negative breast cancer. Cancer Res. 2013;73(22):6711–21. doi:10.1158/0008-5472.CAN-13-1794.
Koncz G, Hancz A, Chakrabandhu K, Gogolak P, Kerekes K, Rajnavolgyi E, et al. Vesicles released by activated T cells induce both Fas-mediated RIP-dependent apoptotic and Fas-independent nonapoptotic cell deaths. J Immunol. 2012;189(6):2815–23. doi:10.4049/jimmunol.1102827.
Barnhart BC, Legembre P, Pietras E, Bubici C, Franzoso G, Peter ME. CD95 ligand induces motility and invasiveness of apoptosis-resistant tumor cells. EMBO J. 2004;23(15):3175–85. doi:10.1038/sj.emboj.7600325.
Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, et al. CD95 promotes tumour growth. Nature. 2010;465(7297):492–6. doi:10.1038/nature09075.
LA OR, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461(7264):659–63. doi:10.1038/nature08402.
Kern P, Dietrich M, Hemmer C, Wellinghausen N. Increased levels of soluble Fas ligand in serum in Plasmodium falciparum malaria. Infect Immun. 2000;68(5):3061–3.
Sugita S, Taguchi C, Takase H, Sagawa K, Sueda J, Fukushi K, et al. Soluble Fas ligand and soluble Fas in ocular fluid of patients with uveitis. Br J Ophthalmol. 2000;84(10):1130–4.
Abe R, Shimizu T, Shibaki A, Nakamura H, Watanabe H, Shimizu H. Toxic epidermal necrolysis and Stevens-Johnson syndrome are induced by soluble Fas ligand. Am J Pathol. 2003;162(5):1515–20. doi:10.1016/S0002-9440(10)64284-8.
Holm AM, Tjonnfjord G, Yndestad A, Beiske K, Muller F, Aukrust P, et al. Polyclonal expansion of large granular lymphocytes in common variable immunodeficiency - association with neutropenia. Clin Exp Immunol. 2006;144(3):418–24. doi:10.1111/j.1365-2249.2006.03086.x.
Nabipour I, Kalantarhormozi M, Assadi M, Jafari SM, Gharibi M, Ahmadi E, et al. Influence of levothyroxine treatment on serum levels of soluble Fas (CD95) and Fas Ligand (CD95L) in chronic autoimmune hypothyroidism. Endocrine. 2010;38(3):406–11. doi:10.1007/s12020-010-9401-x.
Paunel-Gorgulu A, Flohe S, Scholz M, Windolf J, Logters T. Increased serum soluble Fas after major trauma is associated with delayed neutrophil apoptosis and development of sepsis. Crit Care. 2011;15(1):R20. doi:10.1186/cc9965.
D’Agostino S, Salamone M, Di Liegro I, Vittorelli ML. Membrane vesicles shed by oligodendroglioma cells induce neuronal apoptosis. Int J Oncol. 2006;29(5):1075–85.
Bergmann C, Strauss L, Wieckowski E, Czystowska M, Albers A, Wang Y, et al. Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression. Head Neck. 2009;31(3):371–80. doi:10.1002/hed.20968.
Arbuckle E, Langlois NE, Eremin O, Heys SD. Evidence for Fas counter attack in vivo from a study of colorectal cancer. Oncol Rep. 2000;7(1):45–7.
Debatin KM. CD95 system counter attack and prognostic impact. Onkologie. 2006;29(8-9):358–9. doi:10.1159/000094698.
Hefler L, Mayerhofer K, Nardi A, Reinthaller A, Kainz C, Tempfer C. Serum soluble Fas levels in ovarian cancer. Obstet Gynecol. 2000;96(1):65–9.
Furuya Y, Nagakawa O, Fuse H. Prognostic significance of serum soluble Fas level and its change during regression and progression of advanced prostate cancer. Endocr J. 2003;50(5):629–33.
Abbasova SG, Vysotskii MM, Ovchinnikova LK, Obusheva MN, Digaeva MA, Britvin TA, et al. Cancer and soluble FAS. Bull Exp Biol Med. 2009;148(4):638–42.
Aguilar-Lemarroy A, Romero-Ramos JE, Olimon-Andalon V, Hernandez-Flores G, Lerma-Diaz JM, Ortiz-Lazareno PC, et al. Apoptosis induction in Jurkat cells and sCD95 levels in women’s sera are related with the risk of developing cervical cancer. BMC Cancer. 2008;8:99. doi:10.1186/1471-2407-8-99.
Acknowledgments
VOA is grateful for the scholarship obtained from CONACyT Mexico. We are very grateful to Leticia Ramos-Zavala for her efficient technical support in the laboratory. This work was supported by the Fondo de Investigación en Salud, IMSS (grants FIS/IMSS/PROT/G10/874 to AAL and FIS/IMSS/PROT/G09/744 to LFJS).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Vicente Olimón-Andalón and Adriana Aguilar-Lemarroy participated equally.
Rights and permissions
About this article
Cite this article
Olimón-Andalón, V., Aguilar-Lemarroy, A., Ratkovich-González, S. et al. Proapoptotic CD95L levels in normal human serum and sera of breast cancer patients. Tumor Biol. 36, 3669–3678 (2015). https://doi.org/10.1007/s13277-014-3005-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-3005-7