Abstract
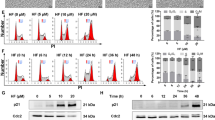
Timosaponin AIII (TAIII) is a steroidal saponin isolated from Anemarrhena asphodeloides that has been shown to inhibit cell growth and induce apoptosis in cancer. However, the effect of TAIII on acute myeloid leukemia (AML) remains unclear. Here, the molecular mechanism by which TAIII-induced apoptosis affects human AML cells was investigated. The results showed that TAIII significantly inhibited cell proliferation of four AML cell lines (MV4-11, U937, THP-1, and HL-60). Furthermore, TAIII induced apoptosis of HL-60 cells through caspase-3, caspase-8, and caspase-9 activations and PARP cleavage in a dose- and time-dependent manner. Moreover, Western blot analysis also showed that TAIII increased phosphorylation of JNK1/2 and p38 MAPK in a dose-dependent manner. Inhibition of JNK1/2 by specific inhibitors significantly abolished the TAIII-induced activation of the caspase-8. Taken together, our results suggest that TAIII induces HL-60 cell apoptosis through JNK1/2 pathways and could serve as a potential additional chemotherapeutic agent for treating AML.




Similar content being viewed by others
References
Ravindranath MH, Ramasamy V, Moon S, Ruiz C, Muthugounder S. Differential growth suppression of human melanoma cells by tea (Camellia sinensis) epicatechins (ECG, EGC and EGCG). Evid Based Complement Alternat Med. 2009;6:523–30.
Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–52.
Shoemaker M, Hamilton B, Dairkee SH, Cohen I, Campbell MJ. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother Res. 2005;19:649–51.
Kang YJ, Chung HJ, Nam JW, Park HJ, Seo EK, Kim YS, et al. Cytotoxic and antineoplastic activity of timosaponin A-III for human colon cancer cells. J Nat Prod. 2011;74:701–6.
Takeda Y, Togashi H, Matsuo T, Shinzawa H, Takeda Y, Takahashi T. Growth inhibition and apoptosis of gastric cancer cell lines by Anemarrhena asphodeloides Bunge. J Gastroenterol. 2001;36:79–90.
Tsai CH, Yang CW, Wang JY, Tsai YF, Tseng LM, King KL, et al. Timosaponin AIII suppresses hepatocyte growth factor-induced invasive activity through sustained ERK activation in breast cancer MDA-MB-231 cells. Evid Based Complement Alternat Med. 2013;2013:421051.
Lok CN, Sy LK, Liu F, Che CM. Activation of autophagy of aggregation-prone ubiquitinated proteins by timosaponin A-III. J Biol Chem. 2011;286:31684–96.
King FW, Fong S, Griffin C, Shoemaker M, Staub R, Zhang YL, et al. Timosaponin AIII is preferentially cytotoxic to tumor cells through inhibition of mTOR and induction of ER stress. PLoS One. 2009;4:e7283.
Lee B, Jung K, Kim DH. Timosaponin AIII, a saponin isolated from Anemarrhena asphodeloides, ameliorates learning and memory deficits in mice. Pharmacol Biochem Behav. 2009;93:121–7.
Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47.
Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–63.
Bishop JF. The treatment of adult acute myeloid leukemia. Semin Oncol. 1997;24:57–69.
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J, Zhang S, et al. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS One. 2012;7:e46853.
Kang SH, Jeong SJ, Kim SH, Kim JH, Jung JH, Koh W, et al. Icariside II induces apoptosis in U937 acute myeloid leukemia cells: role of inactivation of STAT3-related signaling. PLoS One. 2012;7:e28706.
Yang SF, Chen MK, Hsieh YS, Yang JS, Zavras AI, Hsieh YH, et al. Antimetastatic effects of Terminalia catappa L. on oral cancer via a down-regulation of metastasis-associated proteases. Food Chem Toxicol. 2010;48:1052–8.
Hsiao PC, Hsieh YH, Chow JM, Yang SF, Hsiao M, Hua KT, et al. Hispolon induces apoptosis through JNK1/2-mediated activation of a caspase-8, -9, and -3-dependent pathway in acute myeloid leukemia (AML) cells and inhibits AML xenograft tumor growth in vivo. J Agric Food Chem. 2013;61:10063–73.
Chen T, Wong YS. Selenocystine induces S-phase arrest and apoptosis in human breast adenocarcinoma MCF-7 cells by modulating ERK and Akt phosphorylation. J Agric Food Chem. 2008;56:10574–81.
Azijli K, Yuvaraj S, van Roosmalen I, Flach K, Giovannetti E, Peters GJ, et al. MAPK p38 and JNK have opposing activities on TRAIL-induced apoptosis activation in NSCLC H460 cells that involves RIP1 and caspase-8 and is mediated by Mcl-1. Apoptosis. 2013;18:851–60.
Haydn JM, Hufnagel A, Grimm J, Maurus K, Schartl M, Meierjohann S. The MAPK pathway as an apoptosis enhancer in melanoma. Oncotarget. 2014;5:5040–53.
Lee C, Zhang Q, Kozlowski J, Brendler C, Soares MB, Dash A, et al. Natural products and transforming growth factor-beta (TGF-β) signaling in cancer development and progression. Curr Cancer Drug Targets. 2013;13:500–5.
Chien MH, Ying TH, Hsieh YS, Chang YC, Yeh CM, Ko JL, et al. Dioscoreanipponica Makino inhibits migration and invasion of human oral cancer HSC-3 cells by transcriptional inhibition of matrix metalloproteinase-2 through modulation of CREB and AP-1 activity. Food Chem Toxicol. 2012;50:558–66.
Li X, Wang H. Chinese herbal medicine in the treatment of chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:276–81.
Jia W, Gao W, Tang L. Antidiabetic herbal drugs officially approved in China. Phytother Res. 2003;17:1127–34.
Zhou F, Hao YF, Chen Y, Wang T. Chinese herbal medicine in treatment of polyhydramnios:a meta-analysis and systematic review. Chin Med Sci J. 2013;28:72–81.
Wei S, Fukuhara H, Chen G, Kawada C, Kurabayashi A, Furihata M, et al. Terrestrosin D, a steroidal saponin from Tribulus terrestris L., inhibits growth and angiogenesis of human prostate cancer in vitro and in vivo. Pathobiology. 2014;81:123–32.
Jagadeesan J, Nandakumar N, Rengarajan T, Balasubramanian MP. Diosgenin, a steroidal saponin, exhibits anticancer activity by attenuating lipid peroxidation via enhancing antioxidant defense system during NMU-induced breast carcinoma. J Environ Pathol Toxicol Oncol. 2012;31:121–9.
Sun Z, Huang X, Kong L. A new steroidal saponin from the dried stems of Asparagus officinalis L. Fitoterapia. 2010;81:210–3.
Wang N, Feng Y, Zhu M, Siu FM, Ng KM, Che CM. A novel mechanism of XIAP degradation induced by timosaponin AIII in hepatocellular carcinoma. Biochim Biophys Acta. 1833;2013:2890–9.
Sy LK, Yan SC, Lok CN, Man RY, Che CM. Timosaponin A-III induces autophagy preceding mitochondria-mediated apoptosis in HeLa cancer cells. Cancer Res. 2008;68:10229–37.
Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–30.
Kim HS, Lee MS. Essential role of STAT1 in caspase-independent cell death of activated macrophages through the p38 mitogen-activated protein kinase/STAT1/reactive oxygen species pathway. Mol Cell Biol. 2005;25:6821–33.
Chinkwo KA. Sutherlandia frutescens extracts can induce apoptosis in cultured carcinoma cells. J Ethnopharmacol. 2005;98:163–70.
Cai J, Liu M, Wang Z, Ju Y. Apoptosis induced by dioscin in Hela cells. Biol Pharm Bull. 2002;25:193–6.
Hsieh MJ, Tsai TL, Hsieh YS, Wang CJ, Chiou HL. Dioscin-induced autophagy mitigates cell apoptosis through modulation of PI3K/Akt and ERK and JNK signaling pathways in human lung cancer cell lines. Arch Toxicol. 2013;87:1927–37.
Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55.
Spencer SL, Sorger PK. Measuring and modeling apoptosis in single cells. Cell. 2011;144:926–39.
Dai Y, Yu C, Singh V, Tang L, Wang Z, McInistry R, et al. Pharmacological inhibitors of the mitogen-activated protein kinase (MAPK) kinase/MAPK cascade interact synergistically with UCN-01 to induce mitochondrial dysfunction and apoptosis in human leukemia cells. Cancer Res. 2001;61:5106–15.
Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80.
Park SJ, Kim IS. The role of p38 MAPK activation in auranofin-induced apoptosis of human promyelocytic leukaemia HL-60 cells. Br J Pharmacol. 2005;146:506–13.
Yi L, Ji XX, Lin M, Tan H, Tang Y, Wen L, et al. Diallyl disulfide induces apoptosis in human leukemia HL-60 cells through activation of JNK mediated by reactive oxygen. Pharmazie. 2010;65:693–8.
Su JL, Lin MT, Hong CC, Chang CC, Shiah SG, Wu CW, et al. Resveratrol induces FasL-related apoptosis through Cdc42 activation of ASK1/JNK-dependent signaling pathway in human leukemia HL-60 cells. Carcinogenesis. 2005;26:1–10.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hsin-Lien Huang and Whei-Ling Chiang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, HL., Chiang, WL., Hsiao, PC. et al. Timosaponin AIII mediates caspase activation and induces apoptosis through JNK1/2 pathway in human promyelocytic leukemia cells. Tumor Biol. 36, 3489–3497 (2015). https://doi.org/10.1007/s13277-014-2985-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2985-7