Abstract
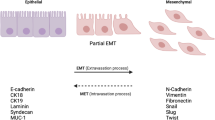
Hypoxia can induce HIF-1α expression and promote the epithelial-mesenchymal transition (EMT) and invasion of cancer cells. However, their mechanisms remain unclear. The objective of this study was to evaluate the role of Gli-1, an effector of the Hedgehog pathway, in the hypoxia-induced EMT and invasion of breast cancer cells. Human breast cancer MDA-MB-231 cells were transfected with HIF-1α or Gli-1-specific small interfering RNA (siRNA) and cultured under a normoxic or hypoxic condition. The relative levels of HIF-1α, Gli-1, E-cadherin, and vimentin in the cells were characterized by quantitative RT-PCR and Western blot assays, and the invasion of MDA-MB-231 cells was determined. Data was analyzed by Student T test, one-way ANOVA, and post hoc LSD test or Mann-Whitney U when applicable. We observed that hypoxia significantly upregulated the relative levels of vimentin expression, but downregulated E-cadherin expression and promoted the invasion of MDA-MB-231 cells, associated with upregulated HIF-1α translation and Gil-1 expression. Knockdown of HIF-1α mitigated hypoxia-modulated Gil-1, vimentin and E-cadherin expression, and invasion of MDA-MB-231 cells. Knockdown of Gil-1 did not significantly change hypoxia-upregulated HIF-1α translation but completely eliminated hypoxia-modulated vimentin and E-cadherin expression and invasion of MDA-MB-231 cells. These data indicate that Gil-1 is crucial for hypoxia-induced EMT and invasion of breast cancer cells and may be a therapeutic target for intervention of breast cancer metastasis.




Similar content being viewed by others
References
Benson JR, Jatoi I. The global breast cancer burden. Future Oncol. 2012;6(8):697–702.
Youlden DR, Cramb SM, Dunn NA, et al. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;3(36):237–48.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;6(119):1420–8.
Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;5(139):871–90.
Wang Y, Zhou BP. Epithelial-mesenchymal transition—a hallmark of breast cancer metastasis. Cancer Hallm. 2013;1(1):38–49.
Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;12(112):1776–84.
Michieli P. Hypoxia, angiogenesis and cancer therapy: to breathe or not to breathe. Cell Cycle. 2009;20(8):3291–6.
Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;4(3):347–61.
Esteban MA, Tran MG, Harten SK, et al. Regulation of E-cadherin expression by VHL and hypoxia-inducible factor. Cancer Res. 2006;7(66):3567–75.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;10(3):721–32.
Cheng ZX, Sun B, Wang SJ, et al. Nuclear factor-kappaB-dependent epithelial to mesenchymal transition induced by HIF-1alpha activation in pancreatic cancer cells under hypoxic conditions. PLoS ONE. 2011;8(6):e23752.
Imai T, Horiuchi A, Wang C, et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;4(163):1437–47.
Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;6(5):437–48.
Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;4(8):S62-7.
Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;1(2):38–47.
Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;4(127):679–95.
Harmon EB, Ko AH, Kim SK. Hedgehog signaling in gastrointestinal development and disease. Curr Mol Med. 2002;1(2):67–82.
Bale AE, Yu KP. The hedgehog pathway and basal cell carcinomas. Hum Mol Genet. 2001;7(10):757–62.
Jeng KS, Sheen IS, Jeng WJ, et al. High expression of Sonic Hedgehog signaling pathway genes indicates a risk of recurrence of breast carcinoma. Onco Targets Ther. 2013;7:79–86.
Ten HA, Bektas N, Von SS, et al. Expression of the glioma-associated oncogene homolog (GLI) 1 in human breast cancer is associated with unfavourable overall survival. BMC Cancer. 2009;9:298.
Lei J, Ma J, Ma Q, et al. Hedgehog signaling regulates hypoxia induced epithelial to mesenchymal transition and invasion in pancreatic cancer cells via a ligand-independent manner. Mol Cancer. 2013;12:66.
Aleffi S, Petrai I, Bertolani C, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;6(42):1339–48.
Novo E, Cannito S, Zamara E, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;6(170):1942–53.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;6(3):1101–8.
Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;5(17):548–58.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;4(9):265–73.
Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;3(270):1230–7.
Wang GL, Jiang BH, Rue EA, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;12(92):5510–4.
Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;5516(292):464–8.
Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;5516(292):468–72.
Yu F, White SB, Zhao Q, et al. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;17(98):9630–5.
Krishnamachary B, Zagzag D, Nagasawa H, et al. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;5(66):2725–31.
Chen J, Imanaka N, Chen J, et al. Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion. Br J Cancer. 2010;2(102):351–60.
Wang G, Zhang Z, Xu Z, et al. Activation of the sonic hedgehog signaling controls human pulmonary arterial smooth muscle cell proliferation in response to hypoxia. Biochim Biophys Acta. 2010;12(1803):1359–67.
Bijlsma MF, Groot AP, Oduro JP, et al. Hypoxia induces a hedgehog response mediated by HIF-1alpha. J Cell Mol Med. 2009;8B(13):2053–60.
Sims-Mourtada J, Yang D, Tworowska I, et al. Detection of canonical hedgehog signaling in breast cancer by 131-iodine-labeled derivatives of the sonic hedgehog protein. J Biomed Biotechnol. 2012; 2012: 639562.
Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;5285(274):255–9.
Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;3(87):553–63.
Stone DM, Hynes M, Armanini M, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;6605(384):129–34.
Li X, Ma Q, Duan W, et al. Paracrine sonic hedgehog signaling derived from tumor epithelial cells: a key regulator in the pancreatic tumor microenvironment. Crit Rev Eukaryot Gene Expr. 2012;2(22):97–108.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianjun Lei and Lin Fan made equal contributions to the study and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Lei, J., Fan, L., Wei, G. et al. Gli-1 is crucial for hypoxia-induced epithelial-mesenchymal transition and invasion of breast cancer. Tumor Biol. 36, 3119–3126 (2015). https://doi.org/10.1007/s13277-014-2948-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2948-z