Abstract
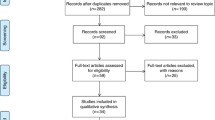
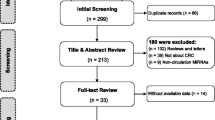
MicroRNAs (miRNAs) are small non-coding RNAs which regulate gene expressions post-transcriptionally. Nowadays, various miRNAs have been found to be sensitive and specific biomarkers for the early diagnosis of colorectal cancer (CRC); however, there are different, even conflicting results in different publications concerning the diagnostic accuracy of miRNA. Therefore, we aim to conduct a meta-analysis of the relevant publications to comprehensively evaluate the diagnostic value of miRNAs in CRC detection. Several public databases such as PubMed, Embase, and Google Scholar were retrieved up to July 13, 2014. Sensitivity was applied to plot the summary receiver operator characteristic (SROC) curve against specificity. The area under the SROC curve (AUC) was calculated to assess the classified effects. STATA 12.0 software was used to perform all statistic analyses. A total of 29 articles, including 80 studies, were involved in our meta-analysis, 55 of which focus on single-miRNA assays and the other 25 on multiple-miRNA assays. Our results suggested that multiple-miRNA assays show a better diagnostic accuracy compared with single-miRNA assays. In addition, blood-based miRNA assays were more accurate than feces-based miRNA assays in CRC diagnosis. Our results also showed that miRNA diagnosis appear to be more accurate in Asians than in Caucasians. However, further researches are needed to validate our results and the feasibility of miRNAs as biomarkers in routine clinical diagnosis of CRC.




Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi:10.1002/ijc.25516.
Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365:153–65. doi:10.1016/S0140-6736(05)17706-X.
Wu CW, Ng SC, Dong Y, Tian L, Ng SS, Leung WW, et al. Identification of microRNA-135b in stool as a potential noninvasive biomarker for colorectal cancer and adenoma. Clin Cancer Res. 2014. doi:10.1158/1078-0432.CCR-13-1750.
Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005;129:422–8. doi:10.1016/j.gastro.2005.05.056.
Wu XD, Song YC, Cao PL, Zhang H, Guo Q, Yan R, et al. Detection of miR-34a and miR-34b/c in stool sample as potential screening biomarkers for noninvasive diagnosis of colorectal cancer. Med Oncol. 2014;31:1–6.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi:10.1038/nrg1379.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi:10.7326/0003-4819-155-8-201110180-00009.
Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi:10.1136/bmj.327.7414.557.
Yang X, Guo Y, Du Y, Yang J, Li S, Liu S, et al. Serum MicroRNA-21 as a diagnostic marker for lung carcinoma: a systematic review and meta-analysis. PLoS ONE. 2014;9:e97460. doi:10.1371/journal.pone.0097460.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–93. doi:10.1016/j.jclinepi.2005.01.016.
Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–81. doi:10.1136/gut.2008.167817.
Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–26.
Pu XX, Huang GL, Guo HQ, Guo CC, Li H, Ye S, et al. Circulating miR-221 directly amplified from plasma is a potential diagnostic and prognostic marker of colorectal cancer and is correlated with p53 expression. J Gastroenterol Hepatol. 2010;25:1674–80. doi:10.1111/j.1440-1746.2010.06417.x.
Kanaan Z, Rai SN, Eichenberger MR, Roberts H, Keskey B, Pan J, et al. Plasma MiR-21: a potential diagnostic marker of colorectal cancer. Ann Surg. 2012;256:544–51.
Chen X, Hu Z, Wang W, Ba Y, Ma L, Zhang C, et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int J Cancer. 2012;130:1620–8. doi:10.1002/ijc.26177.
Wang Q, Huang Z, Ni S, Xiao X, Xu Q, Wang L, et al. Plasma miR-601 and miR-760 are novel biomarkers for the early detection of colorectal cancer. PLoS ONE. 2012;7:e44398. doi:10.1371/journal.pone.0044398.
Feng L, Pang Z, Sha S, Wu B, Xu F, Yin SP. The value of diagnosis and prognosis prediction of serum miR-29a and miR-92a for colorectal cancer. Chin J Clin Oncol Rehabil. 2013:1313–1315.
Giraldez MD, Lozano JJ, Ramirez G, Hijona E, Bujanda L, Castells A, et al. Circulating microRNAs as biomarkers of colorectal cancer: results from a genome-wide profiling and validation study. Clin Gastroenterol Hepatol. 2013;11:681–688.e683.
Kanaan Z, Roberts H, Eichenberger MR, Billeter A, Ocheretner G, Pan J, et al. A plasma microRNA panel for detection of colorectal adenomas: a step toward more precise screening for colorectal cancer. Ann Surg. 2013;258:400–8. doi:10.1097/SLA.0b013e3182a15bcc.
Liu GH, Zhou ZG, Chen R, Wang MJ, Zhou B, Li Y, et al. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour Biol. 2013;34:2175–81. doi:10.1007/s13277-013-0753-8.
Luo X, Stock C, Burwinkel B, Brenner H. Identification and evaluation of plasma microRNAs for early detection of colorectal cancer. PLoS ONE. 2013;8:e62880. doi:10.1371/journal.pone.0062880.
Sheinerman KS, Tsivinsky VG, Umansky SR. Analysis of organ-enriched microRNAs in plasma as an approach to development of universal screening test: feasibility study. J Transl Med. 2013; 11.
Toiyama Y, Takahashi M, Hur K, Nagasaka T, Tanaka K, Inoue Y, et al. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst. 2013;105:849–59.
Wang S, Xiang J, Li Z, Lu S, Hu J, Gao X, et al. A plasma microRNA panel for early detection of colorectal cancer. Int J Cancer. 2013. doi:10.1002/ijc.28136.
Yong FL, Law CW, Wang CW. Potentiality of a triple microRNA classifier: MiR-193a-3p, miR-23a and miR-338-5p for early detection of colorectal cancer. BMC Cancer. 2013; 13.
Zhang GJ, Zhou T, Liu ZL, Tian HP, Xia SS. Plasma miR-200c and miR-18a as potential biomarkers for the detection of colorectal carcinoma. Mol Clin Oncol. 2013;1:379–84.
Hu J, Liu C, Yin Q, Ying M, Li J, Li L, et al. Association between the CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility: a meta-analysis. Mol Genet Genomics. 2014;289:271–7. doi:10.1007/s00438-013-0806-0.
Zanutto S, Pizzamiglio S, Ghilotti M, Bertan C, Ravagnani F, Perrone F, et al. Circulating miR-378 in plasma: a reliable, haemolysis-independent biomarker for colorectal cancer. Br J Cancer. 2014;110:1001–7. doi:10.1038/bjc.2013.819.
Zhang L, Meng L, Fan Z, Liu B, Pei Y, Zhao Z. Expression of plasma miR-106a in colorectal cancer and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:354–7.
Koga Y, Yasunaga M, Takahashi A, Kuroda J, Moriya Y, Akasu T, et al. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res. 2010;3:1435–42.
Kalimutho M, Del Vecchio Blanco G, Di Cecilia S, Sileri P, Cretella M, Pallone F, et al. Differential expression of miR-144*as a novel fecal-based diagnostic marker for colorectal cancer. J Gastroenterol. 2011;46:1391–402.
Koga Y, Yasunaga M, Takahashi A, Kuroda J, Matsumura Y. Fecal RNA test using microRNA expressions of exfoliated colonocytes for colorectal cancer screening. Cancer Res. 2011; 71.
Wu CW, Dong YJ, Ng S, Leung WW, Wong CY, Sung JJ, et al. Identification of a panel of MicroRNAs in stool as screening markers for colorectal cancer. Gastroenterology. 2011;140:S73.
Kuriyama S, Hamaya Y, Yamada T, Sugimoto M, Osawa S, Sugimoto K, et al. Fecal microRNA assays as a marker for colorectal cancer screening. Gastroenterology. 2012;142:S770.
Wu CW, Ng SSM, Dong YJ, Ng SC, Leung WW, Lee CW, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61:739–45.
Koga Y, Yamazaki N, Yamamoto Y, Yamamoto S, Saito N, Kakugawa Y, et al. Fecal miR-106a is a useful marker for colorectal cancer patients with false-negative results in immunochemical fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2013;22:1844–52.
Phua LC, Chue XP, Koh PK, Cheah PY, Chan EC, Ho HK. Global fecal microRNA profiling in the identification of biomarkers for colorectal cancer screening among Asians. Oncol Rep. 2014. doi:10.3892/or.2014.3193.
Andreoli SC, Gasparini NJ, Carvalho GP, Garicochea B, Pogue RE, Andrade RV. Use of microRNAs in directing therapy and evaluating treatment response in colorectal cancer. Einstein (Sao Paulo). 2014;12:256–8.
Schou JV, Rossi S, Jensen BV, Nielsen DL, Pfeiffer P, Hogdall E, et al. miR-345 in metastatic colorectal cancer: a non-invasive biomarker for clinical outcome in non-KRAS mutant patients treated with 3rd line cetuximab and irinotecan. PLoS ONE. 2014;9:e99886. doi:10.1371/journal.pone.0099886.
Zhou XJ, Dong ZG, Yang YM, Du LT, Zhang X, Wang CX. Limited diagnostic value of microRNAs for detecting colorectal cancer: a meta-analysis. Asian Pac J Cancer Prev. 2013;14:4699–704.
Acknowledgments
This work was supported in part by the grant from National Natural Science Foundation of China (Grant # 81272251), a grant for the Innovative Team of Science and Technology, Henan Province, China.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, X., Zhong, J., Ji, Y. et al. The expression and clinical significance of microRNAs in colorectal cancer detecting. Tumor Biol. 36, 2675–2684 (2015). https://doi.org/10.1007/s13277-014-2890-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2890-0