Abstract
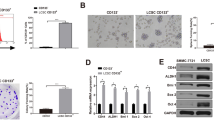
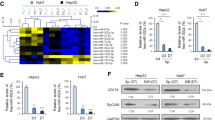
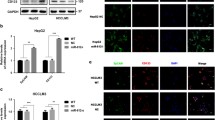
Due to high incidence of invasion and intrahepatic metastasis, hepatocellular carcinoma (HCC) is one of the most aggressive tumors in the world, which is also associated with the acquisition of epithelial-mesenchymal transition (EMT). Increasing evidence suggests that cancer cells with EMT traits share many biological characteristics with cancer stem cells. And miR-200a has been known as a powerful regulator of EMT. Here, we sought to investigate the role of miR-200a in regulation of EMT phenotype of liver cancer stem cells (LCSCs). We used side population (SP) sorting to obtain cancer stem-like cells from HCC cell lines and identified that the SP fraction could be enriched with LCSCs. Then, we detected the expression of miR-200a and EMT makers in SP and non-SP cells. Our results suggested that miR-200a was down-regulated in SP cells, along with relatively low epithelial marker and high mesenchymal marker. In order to find the role of miR-200a in the manipulation of EMT, we transfected miR-200a mimic into LCSCs and found that overexpression of miR-200a resulted in down-regulation of N-cadherin, ZEB2, and vimentin, but up-regulation of E-cadherin. Moreover, overexpression of miR-200a resulted in decreased migration and invasion ability in LCSCs. In conclusion, our study revealed that miR-200a played an important role in linking the characteristics of cancer stem cells with EMT phenotype in HCC, and targeting miR-200a might be an effective strategy to weaken the invasive behavior of LCSCs.





Similar content being viewed by others
References
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–55.
Huo TI et al. The model for end-stage liver disease-based Japan integrated scoring system may have a better predictive ability for patients with hepatocellular carcinoma undergoing locoregional therapy. Cancer. 2006;107(1):141–8.
Olsen SK, Brown RS, Siegel AB. Hepatocellular carcinoma: Review of current treatment with a focus on targeted molecular therapies. Therap Adv Gastroenterol. 2010;3(1):55–66.
Chang WT et al. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: Extending the indication for resection? Surgery. 2012;152(5):809–20.
Roayaie S et al. Resection of hepatocellular cancer </=2 cm: Results from two Western centers. Hepatology. 2013;57(4):1426–35.
Clarke MF et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44.
Dalerba P, Cho RW, Clarke MF. Cancer stem cells: Models and concepts. Annu Rev Med. 2007;58:267–84.
Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea–a paradigm shift. Cancer Res. 2006;66(4):1883–90. discussion 1895–6.
Ponti D et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65(13):5506–11.
Li C et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–7.
Patrawala L et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25(12):1696–708.
Ricci-Vitiani L et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–5.
Zhang N et al. Characterization of a stem-like population in hepatocellular carcinoma MHCC97 cells. Oncol Rep. 2010;23(3):827–31.
Meguro M et al. The molecular pathogenesis and clinical implications of hepatocellular carcinoma. Int J Hepatol. 2011;2011:818672.
Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: Impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21(3):283–96.
Vazquez-Martin A et al. Metformin regulates breast cancer stem cell ontogeny by transcriptional regulation of the epithelial-mesenchymal transition (EMT) status. Cell Cycle. 2010;9(18):3807–14.
Goossens S et al. The EMT regulator Zeb2/Sip1 is essential for murine embryonic hematopoietic stem/progenitor cell differentiation and mobilization. Blood. 2011;117(21):5620–30.
Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–29.
Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54.
Shin SY et al. Functional roles of multiple feedback loops in extracellular signal-regulated kinase and Wnt signaling pathways that regulate epithelial-mesenchymal transition. Cancer Res. 2010;70(17):6715–24.
Takebe N, Warren RQ, Ivy SP. Breast cancer growth and metastasis: Interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Res. 2011;13(3):211.
Yan, H., et al., Inhibitions of epithelial to mesenchymal transition and cancer stem cells-like properties are involved in miR-148a-mediated anti-metastasis of hepatocellular carcinoma. Mol Carcinog, 2013
Mani SA et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Cortez MA et al. MicroRNAs in body fluids–the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–77.
He L et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–33.
Xiao F et al. microRNA-200a is an independent prognostic factor of hepatocellular carcinoma and induces cell cycle arrest by targeting CDK6. Oncol Rep. 2013;30(5):2203–10.
Feng X et al. MiR-200, a new star miRNA in human cancer. Cancer Lett. 2014;344(2):166–73.
Shimono Y et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603.
Bao B et al. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307(1):26–36.
Wu Q et al. MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration and invasion by targeting E-cadherin repressor ZEB2. Gynecol Oncol. 2011;122(1):149–54.
Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14(1):3–9.
Li R et al. MicroRNAs involved in neoplastic transformation of liver cancer stem cells. J Exp Clin Cancer Res. 2010;29:169.
Kang Y, Massague J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118(3):277–9.
Yin S et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120(7):1444–50.
Ishimoto T et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc (−) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400.
Yang W et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68(11):4287–95.
Kimura O et al. Characterization of the epithelial cell adhesion molecule (EpCAM) + cell population in hepatocellular carcinoma cell lines. Cancer Sci. 2010;101(10):2145–55.
Hadnagy A et al. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312(19):3701–10.
Riekstina U et al. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009;5(4):378–86.
Heng JC et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6(2):167–74.
Yu J et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20.
Gregory PA et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601.
Eades G et al. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286(29):25992–6002.
Xia H et al. miR-200a regulates epithelial-mesenchymal to stem-like transition via ZEB2 and beta-catenin signaling. J Biol Chem. 2010;285(47):36995–7004.
Liu J et al. Downregulation of miR-200a induces EMT phenotypes and CSC-like signatures through targeting the beta-catenin pathway in hepatic oval cells. PLoS ONE. 2013;8(11):e79409.
Lu Y et al. MiR-200a inhibits epithelial-mesenchymal transition of pancreatic cancer stem cell. BMC Cancer. 2014;14:85.
Kong D et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS ONE. 2010;5(8):e12445.
Li Y et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69(16):6704–12.
Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126(11):2575–83.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73.
Scheel C et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145(6):926–40.
De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110.
Heddleston JM et al. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8(20):3274–84.
Acknowledgments
We are grateful to Fuqin Zhang who provided technical support. This work was supported by the National Natural Science Foundation of China (grant no. 81172061 and 81401940)
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianlin Wang, Xisheng Yang, and Bai Ruan contributed equally to this work and should be recognized as co-first authors.
Rights and permissions
About this article
Cite this article
Wang, J., Yang, X., Ruan, B. et al. Overexpression of miR-200a suppresses epithelial-mesenchymal transition of liver cancer stem cells. Tumor Biol. 36, 2447–2456 (2015). https://doi.org/10.1007/s13277-014-2856-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2856-2