Abstract
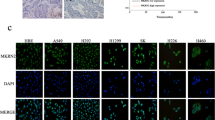
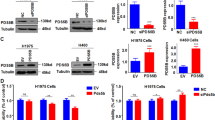
LATS2 (Large tumor suppressor) has been reported to be dys-regulated in several cancer types. However, its function in non-small cell lung cancer (NSCLC) remains poorly understood. Here, it was found that the expression level of LATS2 was decreased in NSCLC tissues. Moreover, forced expression of LATS2 in NSCLC cells inhibited cell growth and migration, while knockdown of the expression of LATS2 promoted the tumorigenicity of NSCLC cells. Mechanistically, LATS2 was found to negatively regulate NF-κB signaling in NSCLC cells. Taken together, our study suggested that down-regulation of LATS2 was very important in the progression of NSCLC, and restoring the function of LATS2 might be a promising therapeutic strategy for NSCLC.





Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
Anagnostou VK, Dimou AT, Botsis T, et al. Molecular classification of nonsmall cell lung cancer using a 4-protein quantitative assay. Cancer. 2012;118(6):1607–18.
Lazar V, Suo C, Orear C, et al. Integrated molecular portrait of non-small cell lung cancers. BMC Med Genom. 2012;6:53.
Goodgame B, Viswanathan A, Zoole J, et al. Risk of recurrence of resected stage I non-small cell lung cancer in elderly patients as compared with younger patients. J Thorac Oncol. 2009;4(11):1370–4.
Broet P, Camilleri-Broet S, Zhang S, et al. Prediction of clinical outcome in multiple lung cancer cohorts by integrative genomics: implications for chemotherapy selection. Cancer Res. 2009;69(3):1055–62.
Campbell JM, Lockwood WW, Buys TP, et al. Integrative genomic and gene expression analysis of chromosome 7 identified novel oncogene loci in non-small cell lung cancer. Genome. 2008;51(12):1032–9.
Kelsey CR, Fornili M, Ambrogi F, et al. Metastasis dynamics for non-small-cell lung cancer: effect of patient and tumor-related factors. Clin Lung Cancer. 2012;14(4):425–32.
Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121(4):1053–63.
Tao W, Zhang S, Turenchalk GS, et al. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat Genet. 1999;21(2):177–81.
Hergovich A, Hemmings BA. Mammalian NDR/LATS protein kinases in hippo tumor suppressor signaling. Biofactors. 2009;35(4):338–45.
Nishioka N, Inoue K, Adachi K, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16(3):398–410.
Muller-Taubenberger A, Kastner PM, Schleicher M, Bolourani P, Weeks G. Regulation of a LATS-homolog by Ras GTPases is important for the control of cell division. BMC Cell Biol. 2012;15(1):25.
Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal. 2012;11(1):31.
Colombani J, Polesello C, Josue F, Tapon N. Dmp53 activates the Hippo pathway to promote cell death in response to DNA damage. Curr Biol. 2006;16(14):1453–8.
Xia H, Qi H, Li Y, et al. LATS1 tumor suppressor regulates G2/M transition and apoptosis. Oncogene. 2002;21(8):1233–41.
Liang Y, Li Y, Li Z, et al. Mechanism of folate deficiency-induced apoptosis in mouse embryonic stem cells: cell cycle arrest/apoptosis in G1/G0 mediated by microRNA-302a and tumor suppressor gene Lats2. Int J Biochem Cell Biol. 2012;44(11):1750–60.
St John MA, Tao W, Fei X, et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21(2):182–6.
Hergovich A. YAP-Hippo signalling downstream of leukemia inhibitory factor receptor: implications for breast cancer. Breast Cancer Res. 2012;14(6):326.
Mojca S, Vid M, Damjan G. LATS2 tumour specific mutations and down-regulation of the gene in non-small cell carcinoma. Lung Cancer. 2009;64:25–262.
Lignitto L, Arcella A, Sepe M, et al. Proteolysis of MOB1 by the ubiquitin ligase praja2 attenuates Hippo signalling and supports glioblastoma growth. Nat Commun. 2012;4:1822.
Li Y, Pei J, Xia H, Ke H, Wang H, Tao W. Lats2, a putative tumor suppressor, inhibits G1/S transition. Oncogene. 2003;22(28):4398–405.
Ishizaki K, Fujimoto J, Kumimoto H, et al. Frequent polymorphic changes but rare tumor specific mutations of the LATS2 gene on 13q11-12 in esophageal squamous cell carcinoma. Int J Oncol. 2002;21(5):1053–7.
Li W, Wang L, Katoh H, Liu R, Zheng P, Liu Y. Identification of a tumor suppressor relay between the FOXP3 and the Hippo pathways in breast and prostate cancers. Cancer Res. 2012;71(6):2162–71.
Lin XY, Zhang XP, Wu JH, Qiu XS, Wang EH. Expression of LATS1 contributes to good prognosis and can negatively regulate YAP oncoprotein in non-small-cell lung cancer. Tumour Biol. 2012;35(7):6435–43.
Ke H, Pei J, Ni Z, et al. Putative tumor suppressor Lats2 induces apoptosis through downregulation of Bcl-2 and Bcl-x(L). Exp Cell Res. 2004;298(2):329–38.
Starczynowski DT, Lockwood WW, Delehouzee S, et al. TRAF6 is an amplified oncogene bridging the RAS and NF-kappaB pathways in human lung cancer. J Clin Invest. 2012;121(10):4095–105.
Nair VS, Gevaert O, Davidzon G, Plevritis SK, West R. NF-kappaB protein expression associates with (18)F-FDG PET tumor uptake in non-small cell lung cancer: a radiogenomics validation study to understand tumor metabolism. Lung Cancer. 2012;83(2):189–96.
Wang LH, Yang JY, Yang SN, et al. Suppression of NF-kappaB signaling and P-glycoprotein function by gambogic acid synergistically potentiates adriamycin-induced apoptosis in lung cancer. Curr Cancer Drug Targets. 2012;14(1):91–103.
Samykutty A, Shetty AV, Dakshinamoorthy G, et al. Piperine, a bioactive component of pepper spice exerts therapeutic effects on androgen dependent and androgen independent prostate cancer cells. PLoS One. 2012;8(6):e65889.
Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2009;91(1):100–17.
Chen X, Su Y, Fingleton B, et al. Increased plasma MMP9 in integrin alpha1-null mice enhances lung metastasis of colon carcinoma cells. Int J Cancer. 2005;116(1):52–61.
Little CD, Nau MM, Carney DN, Gazdar AF, Minna JD. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983;306(5939):194–6.
Aylon Y, Michael D, Shmueli A, Yabuta N, Nojima H, Oren M. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 2006;20(19):2687–700.
Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res. 2008;68(8):2789–94.
Guang W, Ding H, Czinn SJ, Kim KC, Blanchard TG, Lillehoj EP. Muc1 cell surface mucin attenuates epithelial inflammation in response to a common mucosal pathogen. J Biol Chem. 2012;285(27):20547–57.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81201840), the Natural Science Foundation of Shanghai (13ZR1461300), the Health Bureau Foundation of Shanghai (20124Y152), and Chenxing Young Scholarship of Shanghai Jiaotong University.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Feng Yao and Hongcheng Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yao, F., Liu, H., Li, Z. et al. Down-regulation of LATS2 in non-small cell lung cancer promoted the growth and motility of cancer cells. Tumor Biol. 36, 2049–2057 (2015). https://doi.org/10.1007/s13277-014-2812-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2812-1