Abstract
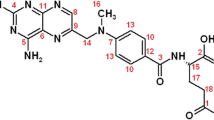
6-Mercaptopurine (6MP) is a well-known purine antimetabolite used to treat childhood acute lymphoblastic leukemia and other diseases. Cancer cells as compared to normal cells are under increased oxidative stress and show high copper level. These differences between cancer cells and normal cells can be targeted to develop effective cancer therapy. Pro-oxidant property of 6MP in the presence of metal ions is not well documented. Redox cycling of Cu(II) to Cu(I) was found to be efficiently mediated by 6MP. We have performed a series of in vitro experiments to demonstrate the pro-oxidant property of 6MP in the presence of Cu(II). Studies on human lymphocytes confirmed the DNA damaging ability of 6MP in the presence of Cu(II). Since 6MP possesses DNA damaging ability by producing reactive oxygen species (ROS) in the presence of Cu(II), it may also possess apoptosis-inducing activity by involving endogenous copper ions. Essentially, this would be an alternative and copper-dependent pathway for anticancer activity of 6MP.








Similar content being viewed by others
References
Lawrance IC. What is left when anti-tumour necrosis factor therapy in inflammatory bowel diseases fails? World J Gastroenterol. 2014;20:1248–58.
Scott FI, Osterman MT. Medical management of Crohn disease. Clin Colon Rectal Surg. 2013;26:67–74.
Frei P, Biedermann L, Nielsen OH, Rogler G. Use of thiopurines in inflammatory bowel disease. World J Gastroenterol. 2013;19:1040–8.
Elion GB. The purine path to chemotherapy. Science. 1989;244:41–7.
Swann PF, Waters TR, Moulton DC, Xu YZ, Zheng Q, Edwards M, et al. Role of post replicative DNA mismatch repair in the cytotoxic action of thioguanine. Science. 1996;273:1109–11.
Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–11.
Serrano J, Palmeira CM, Kuehl DW, Wallace KB. Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochim Biophys Acta. 1999;1411:201–5.
Hug H, Strand S, Grambihler A, Galle J, Hack V, Stremmel W, et al. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J Biol Chem. 1997;272:28191–3.
Miyajima A, Nakashima J, Yoshioka K, Tachibana M, Tazaki H, Murai M. Role of reactive oxygen species in cis-dichlorodiammineplatinum-induced cytotoxicity on bladder cancer cells. Br J Cancer. 1997;76:206–10.
Hadi SM, Bhat SH, Azmi AS, Hanif S, Uzma S, Ullah MF. Oxidative breakage of cellular DNA by plant polyphenols: a putative mechanism for anticancer properties. Semin Cancer Biol. 2007;17:370–6.
Zubair H, Khan HY, Sohail A, Azim S, Ullah MF, Ahmad A, et al. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: putative anticancer mechanism of antioxidants. Cell Death Dis. 2013;4:e660.
Nazeem S, Azmi AS, Hanif S, Kumar KS. Reactive oxygen-dependent dna damage resulting from the oxidation of plumbagin by a copper-redox cycle mechanism: implications for its anticancer properties. Aust-Asian J Cancer. 2008;7:72.
Kela U, Vijayvargiya R. Studies on the mechanism of action of 6-mercaptopurine. Interaction with copper and xanthine oxidase. Biochem J. 1981;193:799–803.
Foye WO. Role of metal-binding in the biological activities of drugs. J Pharm Sci. 1961;50:93–108.
Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2008;35:32–46.
Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, et al. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278:37832–9.
Sastre J, Pallardo FV, Vina J. Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life. 2000;49:427–35.
Li Z, Yang X, Dong S, Li X. DNA breakage induced by piceatannol and copper(II): mechanism and anticancer properties. Oncol Lett. 2012;3:1087–94.
Nakayama T, Kimura T, Kodama M, Nagata C. Generation of hydrogen peroxide and superoxide anion from active metabolites of naphthylamine and amino azo dyes: its possible role in carcinogenesis. Carcinogenesis. 1983;4:765–9.
Quinlan GJ, Gutteridge JMC. Oxygen radical damage to DNA by rifamycin SV and copper ions. Biochem Pharmacol. 1987;36:3629–33.
Ahmad MK, Amani S, Mahmood R. Potassium romated causes cell lysis and induces oxidative stress in human erythrocytes. Environ Toxicol. 2014;29:138–45.
Pool-Zobel BL, Guigas C, Klein RG, Neudecker CH, Renner HW, Schmezer P. Assessment of genotoxic effects by lindane. Food Chem Toxicol. 1993;31:271–83.
Ramanathan A, Das NP, Tan CH. Effects of Ƴ-linolenic acid, flavonoids and vitamins on cytotoxicity and lipid peroxidation. Free Radic Biol Med. 1994;16:43–8.
Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1998;175:184–91.
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–21.
Sarkar B, Roberts EA. The puzzle posed by COMMD1, a newly discovered protein binding Cu(II). Metallomics. 2011;3:20–7.
Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–62.
Mansat-de Mas V, Bezombes C, Quillet-Mary A, Bettaieb A, D’orgeix AD, Laurent G, et al. Implication of radical oxygen species in ceramide generation, c-Jun N-terminal kinase activation and apoptosis induced by daunorubicin. Mol Pharmacol. 1999;56:867–74.
Tsang WP, Chau SP, Kong SK, Fung KP, Kwok TT. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life Sci. 2003;73:2047–58.
Suzuki S, Higuchi M, Proske RJ, Oridate N, Hong WK, Lotan R. Implication of mitochondria-derived reactive oxygen species, cytochrome c and caspase-3 in N-(4-hydroxyphenyl)retinamide-induced apoptosis in cervical carcinoma cells. Oncogene. 1999;18:6380–7.
Asumendi A, Morales MC, Alvarez A, Arechaga J, Perez-Yarza G. Implication of mitochondria-derived ROS and cardiolipin peroxidation in N-(4 hydroxyphenyl) retinamide-induced apoptosis. Br J Cancer. 2002;86:1951–6.
Mishra A, Awate R, Namrata S, Mishra N, Soni R, Sharma P. Synthesis and characterization of transition metal (Cu, Co, Fe) complexes of 6-methyl-5-arylhydrazono-2 thio-4-oxo-pyrimidine ligand. Phosphorus Sulfur Silicon Relat Elem. 2009;184:2624–35.
Prajda N, Morris HP, Weber G. Imbalance of purine metabolism in hepatomas of different growth rates as expressed in behavior of xanthine oxidase (EC 1.2.3.2). Cancer Res. 1976;36:4639–46.
Sau AK, Mondal MS, Mitra S. Interaction of Cu2+ ion with milk xanthine oxidase. Biochim Biophys Acta. 2001;544:89–95.
Acknowledgments
We thank the Council of Scientific and Industrial Research (C.S.I.R.), New Delhi, India, for the award of Senior Research Fellowship to Sayeed Ur Rehman (File no. 09/112(0470)/2011-EMR1). We are also thankful to the Department of Biochemistry, A.M.U. for providing the necessary facilities.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehman, S.U., Zubair, H., Sarwar, T. et al. Redox cycling of Cu(II) by 6-mercaptopurine leads to ROS generation and DNA breakage: possible mechanism of anticancer activity. Tumor Biol. 36, 1237–1244 (2015). https://doi.org/10.1007/s13277-014-2743-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2743-x