Abstract
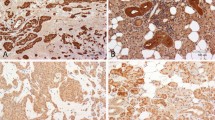
Pleomorphic adenoma (PA) is the most common salivary gland neoplasm. The Hsp27 (HSPB1) is an antiapoptotic protein whose synthesis follows cytotoxic stresses and result in a transient increase in tolerance to subsequent cell injury. Although Hsp27 is expressed in a range of normal tissues and neoplasms, a wide variation in its expression exists among different cells and tissues types. In certain tumours of glandular origin (such as oesophageal adenocarcinomas), the level of Hsp27 is decreased. In the present study, Hsp27 protein levels were evaluated by enzyme-linked immunosorbent assay (ELISA) in a set of 18 fresh PA and 12 normal salivary gland samples. In addition, we tested if Hsp27 protein levels correlated with p53 expression and cell proliferation index, as well as with the transcriptional levels of Bcl-2-associated X protein (BAX), B cell lymphoma 2 (BCL2) and Caspase 3 in PA. We further tested the association between Hsp27 expression and PA tumour size. While all normal salivary gland samples expressed Hsp27 protein, only half of the PA samples expressed it, resulting in a reduced expression of Hsp27 in PA when compared with normal salivary glands (P = 0.003). The expression levels of this protein correlated positively with a higher messenger ribonucleic acid (mRNA) ratio of Bcl2/Bax (R = 0.631; P = 0.01). In conclusion, a decreased Hsp27 protein expression level in PA was found. In addition, Hsp27 levels correlated positively with the Bcl2/Bax mRNA ratio, suggesting an antiapoptotic effect.

Similar content being viewed by others
Abbreviations
- Bax:
-
Bcl-2-associated X protein
- Bcl2:
-
B cell lymphoma 2
- CASP3:
-
Caspase 3
- DNA:
-
Deoxyribonucleic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- Hsp:
-
Heat shock protein
- IHC:
-
Immunohistochemistry
- PA:
-
Pleomorphic adenoma
- PCR:
-
Polymerase chain reaction
- RNA:
-
Ribonucleic acid
References
Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12(10):842–6.
Landry J, Chrétien P, Lambert H, Hickey E, Weber LA. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989;109(1):7–15.
Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56(2):273–9.
Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91(4):1123–59.
Ciocca RD, Adams DJ, Edwards DP, Bjercke RJ, McGuire WL. Distribution of an estrogen-induced protein with a molecular weight of 24,000 in normal and malignant human tissues and cells. Cancer Res. 1983;43(3):1204–10.
Dunn DK, Whelan RDH, Hill B, King RJB. Relationship of HSP27 andoestrogen receptor in hormone sensitive and insensitive cell lines. J Steroid Biochem Mol Biol. 1993;46(4):469–79.
Soldes OS, Kuick RD, Thompson IA, Hughes SJ, Orringer MB, Iannettoni MD, et al. Differential expression of Hsp27 in normal oesophagus, Barrett’s metaplasia and oesophageal adenocarcinomas. Br J Cancer. 1999;79(3–4):595–603.
Lo Muzio L, Leonardi R, Mariggiò MA, Mignogna MD, Rubini C, Vinella A, et al. HSP 27 as possible prognostic factor in patients with oral squamous cell carcinoma. Histol Histopathol. 2004;19(1):119–28.
Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol. 2012;44(10):1622–31.
O’Callaghan-Sunol C, Gabai VL, Sherman MY. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67(24):11779–88.
Xu Y, Diao Y, Qi S, Pan X, Wang Q, Xin Y, et al. Phosphorylated Hsp27 activates ATM-dependent p53 signaling and mediates the resistance of MCF-7 cells todoxorubicin-induced apoptosis. Cell Signal. 2013;25(5):1176–85.
Murray-Zmijewski F, Slee EA, Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol. 2008;9(9):702–12.
Cheng Q, Cao X, Yuan F, Li G, Tong T. Knockdown of WWP1 inhibits growth and induces apoptosis in hepatoma carcinoma cells through the activation of caspase3 and p53. Biochem Biophys Res Commun. 2014;448(3):248–54.
Chandrika BB, Maney SK, Lekshmi SU, Retnabhai ST. Endoplasmic reticulum targeted Bcl2 confers long term cell survival through phosphorylation of heat shockprotein 27. Int J Biochem Cell Biol. 2010;42(12):1984–92.
Havasi A, Li Z, Wang Z, Martin JL, Botla V, Ruchalski K, et al. Hsp27 inhibits Bax activation and apoptosis via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem. 2008;283(18):12305–13.
Rosa DV, Souza RP, Souza BR, Motta BS, Caetano F, Jornada LK, et al. NCS-1 expression in rat brain after electroconvulsive stimulation. Neurochem Res. 2007;32(1):81–5.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Gomes CC, Bernardes VF, Diniz MG, De Marco L, Gomez RS. Anti-apoptotic gene transcription signature of salivary gland neoplasms. BMC Cancer. 2012;12:61.
Gomes CC, Diniz MG, Oliveira CS, Tavassoli M, Odell EW, Gomez RS, et al. Impact of WWOX alterations on p73, ΔNp73, p53, cell proliferation and DNA ploidy in salivary gland neoplasms. Oral Dis. 2011;17(6):564–71.
Vanmuylder N, Evrard L, Daelemans P, Dourov N. Chaperones in the parotid gland: localization of heat shock proteins in human adult salivary glands. Cells Tissues Organs. 2000;167(2–3):199–205.
Amano O, Kudo Y, Shimada M, Wakayama T, Yamamoto M, Iseki S. Transient occurrence of 27 kDa heat-shock protein in the terminal tubule cells during postnatal development of the rat submandibular gland. Anat Rec. 2001;264(4):358–66.
Takahashi-Horiuchi Y, Sugiyama K, Sakashita H, Amano O. Expression of heat shock protein 27 with the transition from proliferation to differentiation of acinar precursor cell in regenerating submandibular gland of rats. Tohoku J Exp Med. 2008;214(3):221–30.
Vanmuylder N, Evrard L, Daelemans P, Van Reck J, Dourov N. Expression of heat shock proteins in salivary gland tumors. Immunohistochemical study of HSP27, HSP70, HSP90, and HSP110: apropos of 50 cases. Ann Pathol. 2000;20(3):190–5.
Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, et al. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111(14):1792–9.
Acknowledgments
This work was supported by grants from the National Council for Scientific and Technological Development (CNPq)/Brazil, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG)/Brazil. Gomez R.S. and Gomes C.C. are research fellows at CNPq. Diniz M.G. is research fellow at Coordination for the Improvement of Higher Education Personnel (CAPES)/Brazil
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Siqueira, E.C., Souza, F.T.A., Diniz, M.G. et al. Hsp27 (HSPB1) differential expression in normal salivary glands and pleomorphic adenomas and association with an increased Bcl2/Bax ratio. Tumor Biol. 36, 213–217 (2015). https://doi.org/10.1007/s13277-014-2634-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2634-1