Abstract
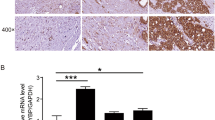
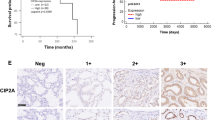
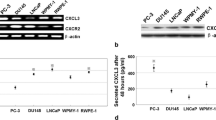
This study aimed to analyze the expression, clinical significance of cyclin G2 (CCNG2) in prostate carcinoma, and the biological effect in its cell line by CCNG2 overexpression. Immunohistochemistry and Western blot were used to analyze CCNG2 protein expression in 85 cases of prostate cancer and normal tissues to study the relationship between CCNG2 expression and clinical factors. CCNG2 lentiviral vector and empty vector were, respectively, transfected into prostate cancer PC-3 cell line. Reverse transcription–polymerase chain reaction (RT-PCR) and Western blot were used to detect the mRNA level and protein of CCNG2. MTT assay and cell cycle were also conducted as to the influence of the upregulated expression of CCNG2 that might be found on PC-3 cells biological effect. The level of CCNG2 protein expression was found to be significantly lower in prostate cancer tissue than normal tissues (P < 0.05). The level of CCNG2 protein expression was not correlated with age, PSA contention, and tumor size (P < 0.05), but it was correlated with lymph node metastasis, clinic stage, and Gleason score (P < 0.05). The result of biological function shown that PC-3 cell transfected CCNG2 had a lower survival fraction, more percentage of the G0/G1 phases, and lower CDK2 protein expression compared with PC-3 cell untransfected CCNG2 (P < 0.05). CCNG2 expression decreased in prostate cancer and correlated significantly with lymph node metastasis, clinic stage, and Gleason score, suggesting that CCNG2 may play important roles as a negative regulator to prostate cancer cell.






Similar content being viewed by others
References
Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics. 2000. CA Cancer J Clin. 2000;50:7–33.
Mettlin CJ, Murphy GP, Rosenthal DS, Menck HR. The national cancer data base report on prostate carcinoma after the peak in incidence rates in the US. Cancer. 1998;83:1679–84.
Ahmed S, Al-Saigh S, Matthews J. FOXA1 is essential for aryl hydrocarbon receptor-dependent regulation of cyclin G2. Mol Cancer Res. 2012;10:636–48.
Bates S, Rowan S, Vousden KH. Characterization of human cyclin G1 and G2: DNA damage inducible genes. Oneogene. 1996;13:1103–9.
Horne MC, Goolsby GL, Donaldson KL, Tran D, Neubauer M, Wahl AF. Cyclin G1 and cyclinG2 comprise a new family of cyclins with contrasting cycle-regulated expression. J Biol Chem. 1996;271:6050–61.
Kim Y, Shintani S, Kohno Y, Zhang R, Wong DT. Cyclin G2 dysregulation in human oral cancer. Cancer Res. 2004;64:8980–6.
Cui XF, Liu AJ, Xu ZM. Expression of Cyclin G2 and its clinical significance in laryngeal squamous cell carcinoma. J Clin Torhinolaryngology. 2009;23:277–9.
Shan G, Shan SG, Zhang XB. Expression and clinical significance of cyclin G1 and cyelinG2 in transitional cell carcinoma of bladder. Chin J Histochem Cytochem. 2009;18:268–72.
Choi MG, Noh JH, An JY, Hong SK, Park SB, Baik YH, et al. Expression levels of cyclinG2, but not cyclin E, correlate with gastric progression. J Surg Res. 2009;157:168–74.
Shi W, Yu KR, Wu GY, Zhang H. Expression of CCNG2 in gastric carcinoma and its relationship with prognosis. Chin J Cell Biol. 2011;33:994–7.
Song YL, Hu GH. CyclinG2: correlation with head and neck neoplasms. Int J Otolaryngology-Head Neck Surg. 2006;30:8–11.
Le XF, Arachehige-Don AS, Mao W, Horne MC, Bast Jr RC. Roles of human epidermal growth factor receptor 2, c-Jun NH2- terminal kinase, PhosPhoinositide 3-kinase, and P7056 kinase pathways in regulation of cyclinG2 expression in human breast cancer cells. Mol Cancer Ther. 2007;6:2843–57.
Martinez GACL, Marqués M, García Z, Campanero MR, Carrera AC. Control of cyclinG2 mRNA expression by forkhead transcription factors: novel mechanism for cell cycle control by phosphoinositide 3-kinase and forkhead. Mol Cell Biol. 2004;4:2181–9.
Chen J, Yusuf L, Andersen HM, Fruman DA. FOXO transcription factors cooperate withδEF1 to activate growth suppressive genes in B-lymphocytes. J Immunol. 2006;76:2711–21.
Xu G, Bernaudo S, Fu G, Lee DY, Yang BB, Peng C. CyclinG2 is degraded through the ubiquitin-proteasome pathway and mediates the antiproliferative effect of activin receptor-like kilase 7. Mol Biol Cell. 2008;9:4968–79.
Cellai C, Laurenzana A, Bianchi E, Sdelci S, Manfredini R, Vannucchi AM, et al. Mechanistic insight intoWEB-2170-induced apoptosis in human acute myelogenous leukemia cells: the crucial role of PTEN. Exp Hematol. 2009;7:1176–85.
van Duijn PW, Ziel-van der Made AC, van der Korput JA, Trapman J. PTEN- mediated Gl cell-cycle arrest in LNCaP prostate cancer cells is associated with altered expression of cell-cycle regulators. Prostate. 2010;70:135–46.
Horne MC, Donaldson KL, Goolsby GL, Tran D, Mulheisen M, Hell JW, et al. Cyclin G2 is up-regulated during growth inhibition and B cell antigen receptor mediated cell cycle arrest. J Biol Chem. 1997;272:12650–61.
Bennin DA, Don AS, Brake T, McKenzie JL, Rosenbaum H, Ortiz L, et al. Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B′ subunits in active complexes and induces nuclear aberrations and a Gl/S phase cell cycle arrest. J Biol Chem. 2002;2779:27449–67.
DonAS A, Dallapiazza RF, Bennin DA, Brake T, Cowan CE, Horne MC. CyclinG2 is a centrosome-associated nucleocytoplasmic shuttling protein that influences microtubule stability and induces a P53-dependent cell cycle arrest. Exp Cell Res. 2006;312:4181–204.
Acknowledgments
This research was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China. The study members, their families, and their friends are thanked for their continuing support.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, D.W., Cheng, Y.J., Jing, S.W. et al. Effect of cyclin G2 on proliferative ability of prostate cancer PC-3 cell. Tumor Biol. 35, 3017–3024 (2014). https://doi.org/10.1007/s13277-013-1389-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1389-4