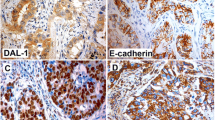
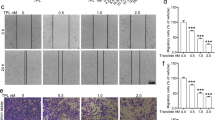
Abstract
The mucin MUC4 is a high molecular weight membrane-bound transmembrane glycoprotein that is frequently detected in invasive and metastatic cancer. The overexpression of MUC4 is associated with increased risks for several types of cancer. However, the functional role of MUC4 is poorly understood in lung adenocarcinoma. Using antisense-MUC4-RNA transfected adenocarcinoma cells, we discovered that the loss of MUC4 expression results in epithelial–mesenchymal transition (EMT). We found morphological alterations and the repression of the epithelial marker E-cadherin in transfected cells. Additionally, the loss of MUC4 caused the upregulation of the mesenchymal marker vimentin compared to control cells. Using a MUC4-knockdown versus control LTEP xenograft mice model (129/sv mice), we also found that EMT happened in lung tissues of MUC4-knockdown-LTEP xenograft mice. Moreover, antisense-MUC4-RNA transfected cells had a significantly increased cellular migration ability in vitro. The loss of MUC4 also occurred in lung adenocarcinoma patients with lymph node metastases. We further investigated MUC4 and found that it plays a critical role in regulating EMT by modulating β-catenin. Taken together, our study reveals a novel role for MUC4 in suppressing EMT and suggests that the assessment of MUC4 may function as a prognostic biomarker and could be a potential therapeutic target for lung adenocarcinoma metastasis.




Similar content being viewed by others
References
Nakamura N et al. Identification of tumour markers and differentiation markers for molecular diagnosis of lung adenocarcinoma. Oncogene. 2006;25(30):4245–55.
Feldser DM et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature. 2010;468(7323):572–5.
Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185(1–3):7–19.
Kyprianou N. ASK-ing EMT not to spread cancer. Proc Natl Acad Sci U S A. 2010;107(7):2731–2.
Shintani Y et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann Thorac Surg. 2011;92(5):1794–804. discussion 1804.
Sato M, Shames DS, Hasegawa Y. Emerging evidence of epithelial-to-mesenchymal transition in lung carcinogenesis. Respirology. 2012;17(7):1048–59.
Kim AN et al. Fyn mediates transforming growth factor-beta1-induced downregulation of E-cadherin in human A549 lung cancer cells. Biochem Biophys Res Commun. 2011;407(1):181–4.
Puisieux A. Role of epithelial-mesenchymal transition in tumour progression. Bull Acad Natl Med. 2009;193(9):2017–32. discussion 2032–4.
Lee JM et al. The epithelial–mesenchymal transition: new insights in signalling, development, and disease. J Cell Biol. 2006;172(7):973–81.
Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Invest. 2009;119(6):1420–8.
Nozawa N et al. Immunohistochemical alpha- and beta-catenin and E-cadherin expression and their clinicopathological significance in human lung adenocarcinoma. Pathol Res Pract. 2006;202(9):639–50.
Kwon KY et al. MUC4 expression in non-small cell lung carcinomas: relationship to tumour histology and patient survival. Arch Pathol Lab Med. 2007;131(4):593–8.
Carraway KL et al. Muc4/sialomucin complex in the mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2001;6(3):323–37.
Singh AP et al. Inhibition of MUC4 expression suppresses pancreatic tumour cell growth and metastasis. Cancer Res. 2004;64(2):622–30.
Ogata S et al. Mucin gene expression in colonic tissues and cell lines. Cancer Res. 1992;52(21):5971–8.
Lopez-Ferrer A et al. MUC4 expression is increased in dysplastic cervical disorders. Hum Pathol. 2001;32(11):1197–202.
Shibahara H et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39(1):220–9.
Majhi PD et al. Pathobiological implications of MUC4 in non-small-cell lung cancer. J Thorac Oncol. 2013;8(4):398–407.
Giuntoli RN et al. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58(23):5546–50.
Weed DT et al. MUC4 and ErbB2 expression in squamous cell carcinoma of the upper aerodigestive tract: correlation with clinical outcomes. Laryngoscope. 2004;114(8 Pt 2 Suppl 101):1–32.
Weed DT et al. MUC4 and ERBB2 expression in major and minor salivary gland mucoepidermoid carcinoma. Head Neck. 2004;26(4):353–64.
Braun J et al. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29(29):4237–44.
Vuoriluoto K et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30(12):1436–48.
Kupferman ME et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene. 2010;29(14):2047–59.
Hiscox S et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118(2):290–301.
Li J, Zhou BP. Activation of beta-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer. 2011;11:49.
Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54.
Wang C et al. The function of SARI in modulating epithelial–mesenchymal transition and lung adenocarcinoma metastasis. PLoS One. 2012;7(9):e38046.
Horn G et al. ERK and PI3K regulate different aspects of the epithelial to mesenchymal transition of mammary tumour cells induced by truncated MUC1. Exp Cell Res. 2009;315(8):1490–504.
Ponnusamy MP et al. MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene. 2010;29(42):5741–54.
Conacci-Sorrell M et al. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signalling, Slug, and MAPK. J Cell Biol. 2003;163(4):847–57.
Asnaghi L et al. E-cadherin negatively regulates neoplastic growth in non-small cell lung cancer: role of Rho GTPases. Oncogene. 2010;29(19):2760–71.
Lee W et al. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature. 2010;465(7297):473–7.
Hata A et al. Erlotinib after Gefitinib failure in relapsed non-small cell lung cancer: clinical benefit with optimal patient selection. Lung Cancer. 2011;74(2):268–73.
Longo F et al. Long-term survival in a smoking Caucasian male patient treated with Gefitinib for spinal cord compression secondary to lung cancer. Onkologie. 2011;34(6):326–8.
Rich AL et al. How do patient and hospital features influence outcomes in small-cell lung cancer in England? Br J Cancer. 2011;105(6):746–52.
Weng JH et al. Pituitary FDG uptake in a patient of lung cancer with bilateral adrenal metastases causing adrenal cortical insufficiency. Clin Nucl Med. 2011;36(8):731–2.
Tsutsumida H et al. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer. 2007;55(2):195–203.
Acknowledgments
We thank Yang Wang (Tianjin Medical University of Cancer Institute and Hospital, Tianjin, China) for the assistance on the revision of the manuscript.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Liuwei Gao and Jun Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, L., Liu, J., Zhang, B. et al. Functional MUC4 suppress epithelial–mesenchymal transition in lung adenocarcinoma metastasis. Tumor Biol. 35, 1335–1341 (2014). https://doi.org/10.1007/s13277-013-1178-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1178-0