Abstract
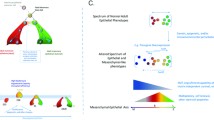
This theoretic paper is an attempt to apply the epigenetic progenitor model of human cancer origin, proposed by Feinberg et al. (Nat Rev Genet 7:21–33, 2006), to the reported phenotype features of invasive breast cancer. The model is based on the idea that expression of estrogen receptors (ER), progesterone receptors (PgR), and HER2 molecules in breast tumors is either remnants of the tissue stem cell from which the tumor has developed or a newly acquired tumor-associated epigenetic feature. HER2 overexpression is considered as an example of the tumor-associated epigenetic changes. The model makes a simple distinction regarding the possible types of ER and PgR expression: the “functional” steroid hormone receptors are inherited from pretumoral tissue stem cells, while the “dysfunctional” steroid hormone receptors are acquired during tumorigenesis from initially ER–PgR-negative cells. In the former, estrogen binding increases the PgR expression while progesterone binding decreases the expression of ER and PgR. Since the estrogen-dependent PgR expression works only in cells with functional ERs, the expected share of tumors with functional ER and PgR receptors is in the model calculated as the squared probability of expressing the PgRs. Reported data from various trials are pooled together to find out phenotype shares (ER+PgR+ makes 62.03 %, ER+PgR− 16.43 %, ER−PgR+ 3.06, and ER−PgR− 18.48 %). By applying the model on these shares, the proposed share of tumors with the functional ER+PgR+ phenotype was 38.48 %, while the share of tumors with the dysfunctional ER+PgR+ was 23.55 %. The presented model suggests that both luminal A and luminal B tumor types are heterogeneous regarding the steroid receptor expression. Some tumors have functional and some have dysfunctional steroid receptors. If these predicted subgroups exist, their detection in the clinical practice might substantially improve treatment options, particularly in the premenopausal setting.

Similar content being viewed by others
References
Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33.
Walker KJ, Price-Thomas JM, Candlish W, Nicholson RI. Influence of the antioestrogen tamoxifen on normal breast tissue. Br J Cancer. 1991;64:764–8.
Jacquemier JD, Hassoun J, Torrente M, Martin PM. Distribution of estrogen and progesterone receptors in healthy tissue adjacent to breast lesions at various stages—immunohistochemical study of 107 cases. Breast Cancer Res Treat. 1990;15:109–17.
Horwitz KB, Koseki Y, McGuire WL. Estrogen control of progesterone receptor in human breast cancer: role of estradiol and antiestrogen. Endocrinology. 1978;103:1742–51.
Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984;2:1102–9.
Lundgren S, Kvinnsland S, Varhaug JE, Utaaker E. The influence of progestins on receptor levels in breast cancer metastasis. Anticancer Res. 1987;7:119–23.
Noguchi S, Yamamoto H, Inaji H, Imaoka S, Koyama H. Influence of tamoxifen-medroxyprogesterone sequential therapy on estrogen and progesterone receptor contents of breast cancer. Jpn J Cancer Res. 1989;80:244–8.
Classen S, Possinger K, Pelka-Fleischer R, Wilmanns W. Effect of onapristone and medroxyprogesterone acetate on the proliferation and hormone receptor concentration of human breast cancer cells. J Steroid Biochem Mol Biol. 1993;45:315–9.
Pujol P, Daures JP, Thezenas S, Guilleux F, Rouanet P, Grenier J. Changing estrogen and progesterone receptor patterns in breast carcinoma during the menstrual cycle and menopause. Cancer. 1998;83:698–705.
Vereide AB, Kaino T, Sager G, Arnes M, Orbo A. Effect of levonorgestrel IUD and oral medroxyprogesterone acetate on glandular and stromal progesterone receptors (PRA and PRB), and estrogen receptors (ER-alpha and ER-beta) in human endometrial hyperplasia. Gynecol Oncol. 2006;101:214–23.
DeCherney AH. The female reproductive system. In: Boron WF, Boulpaep EL, editors. Medical physiology: a cellular and molecular approach. 2nd ed. Philadelphia: Saunders; 2008. p. 1146–69.
Kurbel S. A phase plane graph based model of the ovulatory cycle lacking the “positive feedback” phenomenon. Theor Biol Med Model. 2012;9:35.
Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34:130–62.
Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012;7:CD004143. doi:10.1002/14651858.CD004143.pub4.
O’Toole SA, Beith JM, Millar EK, West R, McLean A, Cazet A, Swarbrick A, Oakes SR. Therapeutic targets in triple negative breast cancer. J Clin Pathol. 2013. doi:10.1136/jclinpath-2012-201361.
Yasui Y, Potter JD. The shape of age-incidence curves of female breast cancer by hormone-receptor status. Cancer Causes Control. 1999;10:431–7.
Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–61.
Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6.
Rakha EA, El-Sayed ME, Green AR, Paish EC, Powe DG, Gee J, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol. 2007;25:4772–8.
Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–9.
Kuhl H, Schneider HP. Progesterone—promoter or inhibitor of breast cancer. Climacteric. 2013. doi:10.3109/13697137.2013.768806.
Keshgegian AA, Cnaan A. Estrogen receptor-negative, progesterone receptor-positive breast carcinoma: poor clinical outcome. Arch Pathol Lab Med. 1996;120(10):970–3.
Kurbel S. In search of triple-negative DCIS: tumor-type dependent model of breast cancer progression from DCIS to the invasive cancer. Tumour Biol. 2013;34:1–7.
Acknowledgments
This theoretic paper was financed through grant 219-2192382-2426 from the Croatian Ministry of Science, Education and Sport.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurbel, S. Model of tumor-associated epigenetic changes of HER2, ER, and PgR expression in invasive breast cancer phenotypes. Tumor Biol. 34, 2011–2017 (2013). https://doi.org/10.1007/s13277-013-0809-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0809-9