Abstract
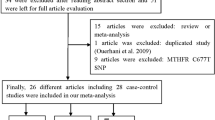
Folate metabolism is thought to play an important role in carcinogenesis through its involvement in both DNA methylation and nucleotide synthesis. The association between the MTHFR Ala222Val polymorphism and bladder cancer has been widely reported, however, in general the data from published studies with individually low statistical power were controversial and underpowered. Hence, we performed a meta-analysis to investigate the association between bladder cancer and MTHFR Ala222Val in different inheritance models. Fourteen studies including a total of 3,570 bladder cancer cases and 3,926 controls for MTHFR rs1801133 polymorphism were included in the meta-analysis. Data were extracted from these studies and odds ratios with corresponding 95 % confidence intervals (95 % CI) were computed to estimate the strength of the association. Overall, the MTHFR Ala222Val polymorphism was not associated with the development of bladder cancer in all genetic models (Ala/Ala vs. Val/Val—OR = 0.961, 95 % CI = 0.763–1.209; Ala/Ala vs. Ala/Val—OR = 0.918, 95 % CI = 0.795–1.060—Ala/Val vs. Val/Val—OR = 1.022, 95 % CI = 0.852–1.227; dominant model—OR = 0.998, 95 % CI = 0.869–1.145; recessive model—OR = 0.921, 95 % CI = 0.794–1.069; Ala allele vs. Val allele—OR = 0.957, 95 % CI = 0.857–1.067). In the stratified analyses, no significant associations were found among different descent populations and sources of controls. Our meta-analysis suggests that the MTHFR Ala222Val polymorphism not contributes to the development of bladder cancer.







Similar content being viewed by others
References
Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics 2001. CA Cancer J Clin. 2001;51:15–36.
Silverman DT. Bladder cancer: biology, diagnosis and management. New York: Oxford University Press; 1999. p. 11–55.
Cohen SM, Shirai T, Steineck G. Epidemiology and etiology of premalignant and malignant urothelial changes. Scand J Urol Nephrol Suppl. 2000;205:105–15.
Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89:630–9.
St Clair DK, Jordan JA, Wan XS, Gairola CG. Protective role of manganese superoxide dismutase against cigarette smoke-induced cytotoxicity. J Toxicol Environ Health. 1994;43:239–49.
García-Closas R, García-Closas M, Kogevinas M, Malats N, Silverman D, Serra C, et al. Food, nutrient and heterocyclic amine intake and the risk of bladder cancer. Eur J Cancer. 2007;43:1731–40.
Mannino DM, Mulinare J, Ford ES, Schwartz J. Tobacco smoke exposure and decreased serum and red blood cell folate levels: data from the third national health and nutrition examination survey. Nicotine Tob Res. 2003;5:357–62.
Tungtrongchitr R, Pongpaew P, Soonthornruengyot M, Viroonudomphol D, Vudhivai N, Tungtrongchitr A, et al. Relationship of tobacco smoking with serum vitamin B-12, folic acid and haematological indices in healthy adults. Public Health Nutr. 2003;6:675–81.
Goyette P, Pai A, Milos R, Frosst P, Tran P, Chen Z, et al. Gene structure of human and mouse methylenetetrahydrofolate reductase (MTHFR). Mamm Genome. 1998;9:652–6.
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3.
Gudnason V, Stansbie D, Scott J, Bowron A, Nicaud V, Humphries S. C677T polymorphism in methynetetrahy-drofolate reductase (MTHFR): its frequency impact on plasma homocysteine concentration in different European populations: EARS group. Atherosclerosis. 1998;136:347–54.
Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methyle-netetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–72.
Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256–66.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–73.
Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;8:15–7.
Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ. 1998;316:469.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Kimura F, Florl AR, Steinhoff C. Polymorphic methyl group metabolism genes in patients with transitional cell carcinoma of the urinary bladder. Mutat Res. 2001;458:49–54.
Sanyal S, Festa F, Sakano S. Polymorphisms in DNA repair and metabolic genes in bladder cancer. Carcinogenesis. 2004;25:729–34.
Lin J, Spitz MR, Wang Y. Polymorphisms of folate metabolic genes and susceptibility to bladder cancer: a case–control study. Carcinogenesis. 2004;25:1639–47.
Moore LE, Wiencke JK, Bates MN. Investigation of genetic polymorphisms and smoking in a bladder cancer case–control study in Argentina. Cancer Lett. 2004;211:199–207.
Karagas MR, Park S, Nelson HH. Methylenetetrahydrofolate reductase (MTHFR) variants and bladder cancer: a population-based case–control study. Int J Hyg Environ Health. 2005;208:321–7.
Moore LE, Malats N, Rothman N. Polymorphisms in one-carbon metabolism and trans-sulfuration pathway genes and susceptibility to bladder cancer. Int J Cancer. 2007;120:2452–8.
Ouerhani S, Oliveira E, Marrakchi R. Methylenetetrahydrofolate reductase and methionine synthase polymorphisms and risk of bladder cancer in a Tunisian population. Cancer Genet Cytogenet. 2007;176:48–53.
Wang M, Zhu H, Fu G. Polymorphisms of methylenetetrahydrofolate reductase and methionine synthase genes and bladder cancer risk: a case–control study with meta-analysis. Clin Exp Med. 2009;9:9–19.
Ouerhani S, Rouissi K, Marrakchi R. Combined effect of NAT2, MTR and MTHFR genotypes and tobacco on bladder cancer susceptibility in Tunisian population. Cancer Detect Prev. 2009;32:395–402.
Rouissi K, Ouerhani S, Oliveira E. Polymorphisms in one-carbon metabolism pathway genes and risk for bladder cancer in a Tunisian population. Cancer Genet Cytogenet. 2009;195:43–53.
Cai DW, Liu XF, Bu RG, Chen XN. Genetic polymorphisms of MTHFR and aberrant promoter hypermethylation of the RASSF1A gene in bladder cancer risk in a Chinese population. J Int Med Res. 2009;37:1882–9.
Safarinejad MR, Shafiei N, Safarinejad S. Genetic susceptibility of methylenetetrahydrofolate reductase (MTHFR) gene C677T, A1298C, and G1793A polymorphisms with risk for bladder transitional cell carcinoma in men. Med Oncol. 2010;28 Suppl 1:S398–412.
Izmirli M, Inandiklioglu N, Abat D, Alptekin D, Demirhan O, Tansug Z, et al. MTHFR gene polymorphisms in bladder cancer in the Turkish population. Asian Pac J Cancer Prev. 2011;12:1833–5.
Fujisawa T, Ikegami H, Kawaguchi Y. Meta-analysis of association of insertion/deletion polymorphism of angiotensin I-converting enzyme gene with diabetic nephropathy and retinopathy. Diabetologia. 1998;41:47–53.
Liwei L, Chunyu L, Ruifa H. Association between manganese superoxide dismutase gene polymorphism and risk of prostate cancer: a meta-analysis. Urology. 2009;74:884–8.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Kai Li and Yong ping Hu contributed equally to this work
Rights and permissions
About this article
Cite this article
Li, K., Hu, Y., Yang, Z. et al. Association between MTHFR Ala222Val (rs1801133) polymorphism and bladder cancer susceptibility: a systematic review and meta-analysis. Tumor Biol. 34, 2565–2572 (2013). https://doi.org/10.1007/s13277-013-0802-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0802-3