Abstract
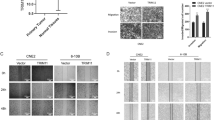
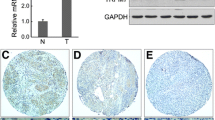
Nasopharyngeal carcinoma (NPC) is a leading malignancy most often reported in endemic areas such as in Southeast Asia and the Mediterranean area. NPC remains as a major challenge for clinical management largely due to its high propensity for cancer invasion, metastasis, and recurrence. Therefore, control of NPC cell motility stands as a major obstacle for successful NPC management. The current study sought to identify a new regulator for NPC cell motility in light of previous data showing a similar role of thyroid receptor interactor protein 6 (TRIP6) in other cancer cell types. Results showed that TRIP6 is up-regulated in NPC cells as compared to normal nasopharyngeal epithelial cells. Moreover, TRIP6 overexpression/knockdown results in significant enhancement/inhibition of NPC cell migration, respectively. Interestingly, data also suggested that TRIP6 Y55E (tyrosine 55 to glutamic acid) mutant can promote cell migration more efficiently than wild type does, while Y55A (tyrosine 55 to alanine) mutant has no effects on cell migration as demonstrated with different methodology. Consistently, we also found that c-Src physically interacts with TRIP6, which suggests its potential role as a TRIP6 kinase. Taken together, these data suggested that TRIP6 is involved in the regulation of NPC cell motility, and phosphorylation of tyrosine 55 residue plays an important regulatory role for this event. These data highlight the importance of TRIP6 as a novel regulator of NPC cell motility, which warrants a good basis for further investigation on the underlying mechanism by which TRIP6 exerts this effect and the pathophysiological role TRIP6 plays in vivo.





Similar content being viewed by others
References
Setton J, Wolden S, Caria N, Lee N. Definitive treatment of metastatic nasopharyngeal carcinoma: report of 5 cases with review of literature. Head & neck. 2012;34:753–7.
Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22:233–44.
Ng WT, Lee MC, Hung WM, Choi CW, Lee KC, Chan OS, et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2011;79:420–8.
Teo P, Leung SF, Yu P, Lee WY, Shiu W. A retrospective comparison between different stage classifications for nasopharyngeal carcinoma. Br J Radiol. 1991;64:901–8.
Sham JS, Cheung YK, Chan FL, Choy D. Nasopharyngeal carcinoma: pattern of skeletal metastases. Br J Radiol. 1990;63:202–5.
Leung SF, Teo PM, Shiu WW, Tsao SY, Leung TW. Clinical features and management of distant metastases of nasopharyngeal carcinoma. J Otolaryngol. 1991;20:27–9.
Yi J, Beckerle MC. The human TRIP6 gene encodes a LIM domain protein and maps to chromosome 7q22, a region associated with tumorigenesis. Genomics. 1998;49:314–6.
Murthy KK, Clark K, Fortin Y, Shen SH, Banville D. ZRP-1, a zyxin-related protein, interacts with the second PDZ domain of the cytosolic protein tyrosine phosphatase hPTP1E. J Biol Chem. 1999;274:20679–87.
Cuppen E, van Ham M, Wansink DG, de Leeuw A, Wieringa B, Hendriks W. The zyxin-related protein TRIP6 interacts with PDZ motifs in the adaptor protein RIL and the protein tyrosine phosphatase PTP-BL. Eur J Cell Biol. 2000;79:283–93.
Petit MM, Crombez KR, Vervenne HB, Weyns N, Van de Ven WJ. The tumor suppressor Scrib selectively interacts with specific members of the zyxin family of proteins. FEBS Lett. 2005;579:5061–8.
Beckerle MC. Zyxin: Zinc fingers at sites of cell adhesion. BioEssays. 1997;19:949–57.
Petit MM, Mols R, Schoenmakers EF, Mandahl N, Van de Ven WJ. Lpp, the preferred fusion partner gene of hmgic in lipomas, is a novel member of the lim protein gene family. Genomics. 1996;36:118–29.
Marie H, Pratt SJ, Betson M, Epple H, Kittler JT, Meek L, et al. The LIM protein Ajuba is recruited to cadherin-dependent cell junctions through an association with alpha-catenin. J Biol Chem. 2003;278:1220–8.
Hervy M, Hoffman L, Beckerle MC. From the membrane to the nucleus and back again: Bifunctional focal adhesion proteins. Curr Opin Cell Biol. 2006;18:524–32.
Gur'ianova OA, Sablina AA, Chumakov PM. Frolova EI: [Down-regulation of TRIP6 expression induces actin cytoskeleton rearrangements in human carcinoma cell lines]. Molekuliarnaia biologiia. 2005;39:905–9.
Xu J, Lai YJ, Lin WC, Lin FT. TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor. J Biol Chem. 2004;279:10459–68.
Huggins CJ, Andrulis IL. Cell cycle regulated phosphorylation of LIMD1 in cell lines and expression in human breast cancers. Cancer Lett. 2008;267:55–66.
Li L, Bin LH, Li F, Liu Y, Chen D, Zhai Z, et al. TRIP6 is a RIP2-associated common signaling component of multiple NF-kappaB activation pathways. Journal of cell science. 2005;118:555–63.
Behrens J, Weidner KM, Frixen UH, Schipper JH, Sachs M, Arakaki N, et al. The role of E-cadherin and scatter factor in tumor invasion and cell motility. EXS. 1991;59:109–26.
Hendrix MJ, Seftor EA, Chu YW, Trevor KT, Seftor RE. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev. 1996;15:507–25.
Lin VT, Lin FT. Trip6: An adaptor protein that regulates cell motility, antiapoptotic signaling and transcriptional activity. Cell Signal. 2011;23:1691–7.
Lai YJ, Lin WC, Lin FT. PTPL1/FAP-1 negatively regulates TRIP6 function in lysophosphatidic acid-induced cell migration. J Biol Chem. 2007;282:24381–7.
Lai YJ, Chen CS, Lin WC. Lin FT: c-Src-mediated phosphorylation of TRIP6 regulates its function in lysophosphatidic acid-induced cell migration. Mol Cell Biol. 2005;25:5859–68.
Chong VF, Ong CK. Nasopharyngeal carcinoma. Eur J Radiol. 2008;66:437–47.
Arango BA, Castrellon AB, Perez CA, Raez LE, Santos ES. Nasopharyngeal carcinoma: alternative treatment options after disease progression. Expert Rev Anticancer Ther. 2010;10:377–86.
26 Lin VT, Lin VY, Lai YJ, Chen CS, Liu K, Lin WC, Lin FT (2013) TRIP6 regulates p27KIP1 to promote tumorigenesis. Mol Cell Biol
Chastre E, Abdessamad M, Kruglov A, Bruyneel E, Bracke M, Di Gioia Y, et al. TRIP6, a novel molecular partner of the MAGI-1 scaffolding molecule, promotes invasiveness. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23:916–28.
Sirvent A, Benistant C, Pannequin J, Veracini L, Simon V, Bourgaux JF, et al. Src family tyrosine kinases-driven colon cancer cell invasion is induced by Csk membrane delocalization. Oncogene. 2010;29:1303–15.
Zhou J, Scholes J, Hsieh JT. Characterization of a novel negative regulator (DOC-2/DAB2) of c-Src in normal prostatic epithelium and cancer. J Biol Chem. 2003;278:6936–41.
Gomes EG, Connelly SF, Summy JM (2013) Targeting the yin and the yang: combined inhibition of the tyrosine kinase c-Src and the tyrosine phosphatase SHP-2 disrupts pancreatic cancer signaling and biology in vitro and tumor formation in vivo. Pancreas
Nam HJ, Im SA, Oh DY, Elvin P, Kim HP, Yoon YK, et al. Antitumor activity of saracatinib (AZD0530), a c-Src/Abl kinase inhibitor, alone or in combination with chemotherapeutic agents in gastric cancer. Mol Cancer Ther. 2013;12:16–26.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fei, J., Li, J., Shen, S. et al. Characterization of TRIP6-dependent nasopharyngeal cancer cell migration. Tumor Biol. 34, 2329–2335 (2013). https://doi.org/10.1007/s13277-013-0780-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-0780-5