Abstract
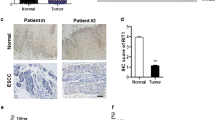
Emerging evidence indicates that RhoE as novel member of the Rho GTPases family plays an essential role in carcinogenesis and tumor progression of human various tumors, but the functional significance of RhoE in human esophageal squamous cell carcinoma (ESCC) is still unclear. In the current study, RhoE expression in ESCC tissues and cells was examined, and the biological functions of RhoE in ESCC cells were determined. The results revealed that RhoE expression at mRNA and protein levels was significantly downregulated in ESCC tissues and cell lines (P < 0.05). RhoE expression was tightly associated with differentiation degree, clinical staging, and lymph node metastasis of the patients with ESCC (P < 0.05), but no significant correlations were found between RhoE expression and gender or age of the patients with ESCC (P > 0.05). Additionally, we found that downregulation of RhoE expression in ESCC cells promoted cell proliferation, cell cycle progression, as well as cell invasion in vitro, and inhibited cell apoptosis. Conversely, upregulation of RhoE expression in ESCC cells inhibited cell proliferation, arrested cell cycle at G0/G1 phase, reduced cell invasion, and promoted cell apoptosis. Furthermore, the downregulation of RhoE expression significantly reduced PTEN level and enhanced pAkt level; however, elevation of RhoE expression markedly increased PTEN level and decreased pAkt level. Stepwise investigations demonstrated that overexpression of RhoE in ESCC cells increased the expressions of p27 and bax proteins but decreased the expressions of cyclin D1 and bcl-2 proteins. These data demonstrate that RhoE may play a driving role in the development and progression of ESCC, and targeting the RhoE may be an effective and feasible approach for treatment of ESCC.







Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–50.
Parkin DM, Bray FI, Devesa SS. Cancer burden in the year, the global picture. Eur J Cancer. 2000;2001(37 Suppl 8):S4–66.
Ekman S, Dreilich M, Lennartsson J, Wallner B, Brattstrom D, Sundbom M, Bergqvist M. Esophageal cancer: current and emerging therapy modalities. Expert Rev Anticancer Ther. 2008;8(9):1433–48.
Xing D, Tan W, Lin D. Genetic polymorphisms and susceptibility to esophageal cancer among Chinese population (review). Oncol Rep. 2003;10(5):1615–23.
Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121(8):1643–58.
Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54(4):594–606.
Lu SM, Su M, Tian DP, Deng WD, Zheng YL, Huang HH, Chen MH, Li XY. Characterization of one newly established esophageal cancer cell line CSEC from a high-incidence area in China. Dis Esophagus. 2008;21(4):309–15.
Yang L, Parkin DM, Li L, Chen Y. Time trends in cancer mortality in China: 1987–1999. Int J Cancer. 2003;106(5):771–83.
Enzinger PC, Ilson DH, Kelsen DP. Chemotherapy in esophageal cancer. Semin Oncol. 1999;26(5 Suppl 15):12–20.
Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–27.
Riou P, Villalonga P, Ridley AJ. Rnd proteins: multifunctional regulators of the cytoskeleton and cell cycle progression. Bioessays. 2010;32(11):986–92.
Ongusaha PP, Kim HG, Boswell SA, Ridley AJ, Der CJ, Dotto GP, Kim YB, Aaronson SA, Lee SW. RhoE is a pro-survival p53 target gene that inhibits ROCK I-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006;16(24):2466–72.
Belgiovine C, Frapolli R, Bonezzi K, Chiodi I, Favero F, Mello-Grand M, Dei Tos AP, Giulotto E, Taraboletti G, D’Incalci M, et al. Reduced expression of the ROCK inhibitor Rnd3 is associated with increased invasiveness and metastatic potential in mesenchymal tumor cells. PLoS One. 2010;5(11):e14154.
Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7(1):54–62.
Foster R, Hu KQ, Lu Y, Nolan KM, Thissen J, Settleman J. Identification of a novel human Rho protein with unusual properties: GTPase deficiency and in vivo farnesylation. Mol Cell Biol. 1996;16(6):2689–99.
Zhang C, Zhou F, Li N, Shi S, Feng X, Chen Z, Hang J, Qiu B, Li B, Chang S, et al. Overexpression of RhoE has a prognostic value in non-small cell lung cancer. Ann Surg Oncol. 2007;14(9):2628–35.
Bektic J, Pfeil K, Berger AP, Ramoner R, Pelzer A, Schafer G, Kofler K, Bartsch G, Klocker H. Small G-protein RhoE is underexpressed in prostate cancer and induces cell cycle arrest and apoptosis. Prostate. 2005;64(4):332–40.
Luo H, Dong Z, Zou J, Zeng Q, Wu D, Liu L. Down-regulation of RhoE is associated with progression and poor prognosis in hepatocellular carcinoma. J Surg Oncol 2011. doi:10.1002/jso.23019.
Guasch RM, Scambler P, Jones GE, Ridley AJ. RhoE regulates actin cytoskeleton organization and cell migration. Mol Cell Biol. 1998;18(8):4761–71.
Gadea G, de Toledo M, Anguille C, Roux P. Loss of p53 promotes RhoA-ROCK-dependent cell migration and invasion in 3D matrices. J Cell Biol. 2007;178(1):23–30.
Liu HT, Wang N, Wang X, Li SL. Overexpression of Pim-1 is associated with poor prognosis in patients with esophageal squamous cell carcinoma. J Surg Oncol. 2010;102(6):683–8.
Muskhelishvili L, Latendresse JR, Kodell RL, Henderson EB. Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J Histochem Cytochem. 2003;51(12):1681–8.
Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, Herlyn M, Rustgi AK. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278(3):1824–30.
Li S, Jiao J, Lu Z, Zhang M. An essential role for N-cadherin and beta-catenin for progression in tongue squamous cell carcinoma and their effect on invasion and metastasis of Tca8113 tongue cancer cells. Oncol Rep. 2009;21(5):1223–33.
Lu Z, Liu H, Xue L, Xu P, Gong T, Hou G. An activated Notch1 signaling pathway inhibits cell proliferation and induces apoptosis in human esophageal squamous cell carcinoma cell line EC9706. Int J Oncol. 2008;32(3):643–51.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8.
Gress TM, Muller-Pillasch F, Geng M, Zimmerhackl F, Zehetner G, Friess H, Buchler M, Adler G, Lehrach H. A pancreatic cancer-specific expression profile. Oncogene. 1996;13(8):1819–30.
Akashi H, Han HJ, Iizaka M, Nakamura Y. Growth-suppressive effect of non-steroidal anti-inflammatory drugs on 11 colon-cancer cell lines and fluorescence differential display of genes whose expression is influenced by sulindac. Int J Cancer. 2000;88(6):873–80.
van Groningen JJ, Cornelissen IM, van Muijen GN, Bloemers HP, Swart GW. Simultaneous suppression of progression marker genes in the highly malignant human melanoma cell line BLM after transfection with the adenovirus-5 E1A gene. Biochem Biophys Res Commun. 1996;225(3):808–16.
Trojan L, Schaaf A, Steidler A, Haak M, Thalmann G, Knoll T, Gretz N, Alken P, Michel MS. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res. 2005;25(1A):183–91.
Bektic J, Wrulich OA, Dobler G, Kofler K, Ueberall F, Culig Z, Bartsch G, Klocker H. Identification of genes involved in estrogenic action in the human prostate using microarray analysis. Genomics. 2004;83(1):34–44.
Liu T, Bauskin AR, Zaunders J, Brown DA, Pankhurst S, Russell PJ, Breit SN. Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells. Cancer Res. 2003;63(16):5034–40.
Chen J, Zhou H, Li Q, Qiu M, Li Z, Tang Q, Liu M, Zhu Y, Huang J, Lang N, et al. Epigenetic modification of RhoE expression in gastric cancer cells. Oncol Rep. 2011;25(1):173–80.
Riento K, Villalonga P, Garg R, Ridley A. Function and regulation of RhoE. Biochem Soc Trans. 2005;33(Pt 4):649–51.
Villalonga P, Guasch RM, Riento K, Ridley AJ. RhoE inhibits cell cycle progression and Ras-induced transformation. Mol Cell Biol. 2004;24(18):7829–40.
Poch E, Minambres R, Mocholi E, Ivorra C, Perez-Arago A, Guerri C, Perez-Roger I, Guasch RM. RhoE interferes with Rb inactivation and regulates the proliferation and survival of the U87 human glioblastoma cell line. Exp Cell Res. 2007;313(4):719–31.
Boswell SA, Ongusaha PP, Nghiem P, Lee SW. The protective role of a small GTPase RhoE against UVB-induced DNA damage in keratinocytes. J Biol Chem. 2007;282(7):4850–8.
Li K, Lu Y, Liang J, Luo G, Ren G, Wang X, Fan D. RhoE enhances multidrug resistance of gastric cancer cells by suppressing Bax. Biochem Biophys Res Commun. 2009;379(2):212–6.
Li H, Gao Q, Guo L, Lu SH. The PTEN/PI3K/Akt pathway regulates stem-like cells in primary esophageal carcinoma cells. Cancer Biol Ther. 2011;11(11):950–8.
Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4(3):226–35.
Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, Garcia-Echeverria C, Schultz PG, Reddy VA. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106(1):268–73.
Liu S, Ma X, Ai Q, Huang Q, Shi T, Zhu M, Wang B, Zhang X: NOTCH1 functions as an oncogene by regulating the PTEN/PI3K/AKT pathway in clear cell renal cell carcinoma. Urol Oncol 2011.
Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7(5):812–21.
Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signal. Cell. 1994;78(1):59–66.
Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–8.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81071723), Natural Science Foundation of He’nan Educational Committee (2010A310008), and Key Technologies R & D Program of He’nan Province (112102310699).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Contributors
Hui Zhao, Jianping Yang, and Tianli Fan contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhao, H., Yang, J., Fan, T. et al. RhoE functions as a tumor suppressor in esophageal squamous cell carcinoma and modulates the PTEN/PI3K/Akt signaling pathway. Tumor Biol. 33, 1363–1374 (2012). https://doi.org/10.1007/s13277-012-0384-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0384-5