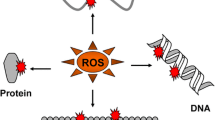
Abstract
The present review deals with the genetic implications of reactive oxygen species (ROS) to enhance horizons of chemophototherapy toward novel approaches for the treatment of various cancers. ROS are species of oxygen which are in a more reactive state than molecular oxygen. ROS play essential roles in vivo such as redox regulation, gene expression, immune response and many other cellular events. ROS generated by anticancer drugs during chemophototherapy may be associated with the activation of signal molecules like PKC, transcription factor NF-kappaB as well as destabilization of mitochondrial membrane inducing the release of apoptosis inducing agents like cytochrome c, resulting in toxicity to cancer cells. Thus, we suggest that anticancer drugs on exposure to light may generate oxidative stress following Fenton-like reaction generating hydroxyl radical. This may get on specific cell cycle receptors which may lead to cell cycle arrest and subsequently cytotoxic death of cancer cells.
Similar content being viewed by others
References
Steiner U, Klein J, Eiser E, Budkowski A, Fetters LJ. Complete wetting from polymer mixtures. Science. 1992;25:1122–9.
Bauerschmidt C, Arrichiello C, Burdak-Rothkamm S, Woodcock M, Hill MA, Stevens DL, Rothkamm K. Cohesin promotes the repair of ionizing radiation-induced DNA double-strand breaks in replicated chromatin. Nucleic Acids Res. 2010;38:477–87.
Mendonca MS, Chin-Sinex H, Gomez-Millan J, Datzman N, Hardacre M, Comerford K, Nakshatri H, Nye M, Benjamin L, Mehta S, Patino F, Sweeney C. Parthenolide sensitizes cells to X-ray-induced cell killing through inhibition of NF-κB and split-dose repair. Radiat Res. 2007;168:689–97.
Tomita M. Involvement of DNA-PK and ATM in radiation- and heat-induced DNA damage recognition and apoptotic cell death. J Radiat Res. 2010;51:493–501.
Renschle MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–40.
Acharya A, Das I, Chandhok D, Saha T. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3:23–34.
Chiharu IK, Toshio S. Regulation of reactive oxygen species in stem cells and cancer stem cells. J Cell Physiol. 2011. doi:10.1002/jcp.22764.
Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–50.
Conklin KA. Cancer chemotherapy and antioxidants. J Nutr. 2004;134:3201S–4.
Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB’, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24:95–9.
Chibber S, Hassan I, Farhan M, Naseem I. In vitro prooxidant action of methotrexate in presence of white light. Photochem Photobiol B: Biol. 2011;104:387–93.
Chibber S, Farhan M, Hassan I, Naseem I. White light-mediated Cu (II)-5FU interaction augments the chemotherapeutic potential of 5-FU: an in vitro study. Tumor Biol. 2011;32:881–92.
Kazutaka H. Fluorometry of singlet oxygen generated via a photosensitized reaction using folic acid and methotrexate. Anal Bioanal Chem. 2002;393:999–1005.
Jaroslav C, Jiøí G, Jiøina M, Marie Š, Jaroslava V, Vìra K, Marie N. Pharmacokinetics and pharmacodynamics of low-dose methotrexate in the treatment of psoriasis. Br J Clin Pharmacol. 2002;54:147–56.
Pascu ML, Brezeanu M, Voicu L, Staicu A, Carstocea B, Pascu RA. 5-Fluorouracil as a photosensitiser. in vivo. 2005;19:215–20.
Hassan I, Chibber S, Naseem I. Ameliorative effect of riboflavin on the cisplatin induced nephrotoxicity and hepatotoxicity under photoillumination. Food Chem Toxicol. 2010;48:2052–8.
Saxton RE, Paiva MB, Lufkin RB, Castro DJ. Laser photochemotherapy: a less invasive approach for treatment of cancer. Semin Surg Oncol. 1995;11:283–9.
Duska LR, Hamblin MR, Miller JL, Hasan T. Combination photoimmunotherapy and cisplatin: effects on human ovarian cancer ex vivo. J Natl Cancer Inst. 1999;91:1557–63.
Skeel RT. Handbook of cancer chemotherapy. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2003. ISBN 0781736293.
Katherine LK, John LA, Jane CW, Elizabeth AC, Deborah S, John ZA, Catarina IK, Patricia AG, Nirmala B, Arnold LP, David PH, Robert HF. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA. 2010;303:1037–45.
Bore C. Antioxidants and radiation therapy. J Nutr. 2004;134:3207S–9.
Wen-Hsiung CJ. Photodynamic treatment induces an apoptotic pathway involving calcium, nitric oxide, p53, p21-activated kinase 2, and c-Jun N-terminal kinase and inactivates survival signal in human umbilical vein endothelial cells. Int J Mol Sci. 2011;12:1041–59.
Al-Ejeh F, Kumar R, Wiegmans A, Lakhan SR, Brown MP, Khanna KK. Harnessing the complexity of DNA-damage response pathways to improve cancer treatment outcomes. Oncogene. 2010;29:6085–98.
Shao C, Folkard M, Held KD, Prise KM. Estrogen enhanced cell–cell signalling in breast cancer cells exposed to targeted irradiation. BMC Cancer. 2008;82:184.
Pandey BN, Mishra KP. Role of membrane oxidative damage and reactive oxygen species in radiation induced apoptotic death in mouse thymocytes. Int J low rad. 2003;1:113–9.
Maity A, McKenna WG, Muschel RJ. The molecular basis for cell cycle delays following ionizing radiation: a review. Radiother Oncol. 1994;31:1–13.
Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A. Platelet derived growth factor-induced H2O2 production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:10527–31K.
Junn E, Lee KN, Ju HR, Han SH, Im JY, Kang HS. Requirement of hydrogen peroxide generation in TGF-beta 1 signal transduction in human lung fibroblast cells: involvement of hydrogen peroxide and Ca2+ in TGF beta 1-induced IL-6 expression. J Immunol. 2000;165:2190–7.
Howes RM. Hydrogen peroxide: a review of a scientifically verifiable omnipresent ubiquitous essentiality of obligate, aerobic, carbon-based life forms. Int J Plastic Surg. 2010;7(1).
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet derived growth factor signal transduction. Science. 1995;270:296–9.
Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–21.
Groen A, Lemeer S, Van der Wijk T, Overvoorde J, Heck AJ, Ostman A, Barford D, Slijper M, Den Hertog J. Differential oxidation of protein-tyrosine phosphatases. J Biol Chem. 2005;280:10298–304.
Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI3-kinase signaling via inactivation of PTEN. EMBO J. 2003;22:5501–10.
Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–31.
Benhar M, Engelberg D, Levitzki A. ROS, stress activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–5.
Li CY, Shan S, Huang Q, Braun RD, Lanzen J, Hu K, Lin P, Dewhirst MW. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–7.
Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300.
Sentürker S, Tschirret-Guth R, Morrow J, Levine R, Shacter E. Induction of apoptosis by chemotherapeutic drugs without generation of reactive oxygen species. Arch Biochem Biophys. 2002;397:262–72.
Hauptlorenz S, Esterbauer H, Moll W, Pumpel R, Schauenstein E, Puschendorf B. Effects of the lipid peroxidation product 4-hydroxynonenal and related aldehydes on proliferation and viability of cultured Ehrlich ascites tumor cells. Biochem Pharmacol. 1985;34:3803–9.
Pizzimenti S, Menegatti E, Berardi D, Toaldo C, Pettazzoni P, Minelli R, Giglioni B, Cerbone A, Dianzani MU, Ferretti C, Barrera G. 4-Hydroxynonenal, a lipid peroxidation product of dietary polyunsaturated fatty acids, has anticarcinogenic properties in colon carcinoma cell lines through the inhibition of telomerase activity. J Nutr Biochem. 2010;21:818–26.
Riahi Y, Cohen G, Shamni O, Sasson S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am J Physiol Endocrinol Metab. 2010;299:E879–86.
Khoschsorur G, Schaur RJ, Schauenstein E, Tillian HM, Reiter M. Intracellular effect of hydroxyalkenals on animal tumors. Z Naturforsch. 1981;36:572–8.
Hussain AR, Ahmed M, Ahmed S, Manogaran P, Platanias LC, Alvi SN, Al-Kuraya KS, Uddin S. Thymoquinone suppresses growth and induces apoptosis via generation of reactive oxygen species in primary effusion lymphoma. Free Radic Biol Med. 2011;50:978–87.
Yael R, Guy C, Ofer S, Shlomo S. Signaling and cytotoxic functions of 4 hydroxyalkenals. AJP–Endo. 2010;299:E879–86.
Shacter E, Williams JA, Hinson RM, Senturker S, Lee YJ. Oxidative stress interferes with cancer chemotherapy: inhibition of lymphoma cell apoptosis and phagocytosis. Blood. 2000;96:307–13.
Lee YJ, Shacter E. Oxidative stress inhibits apoptosis in human lymphoma cells. J Biol Chem. 1999;274:19792–8.
Wilson KP, Black JAF, Thomson JA, et al. Structure and mechanism of interleukin-1β converting enzyme. Nature. 1994;370:270–5.
Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8.
Ali I, Sakhnini N, Naseem I. Hemolysis of human red blood cells by riboflavin–Cu (II) system. Biochemistry (Mosc). 2000;70:1011–4.
Ali I, Naseem I. Hemolysis of human red blood cells by combination of riboflavin and aminophylline. Life Sci. 2002;70:2013–22.
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radical in DNA damage and cancer incidence. J Natl Cancer Inst. 2004;266:37–56.
Acknowledgments
The authors sincerely acknowledge the financial assistance provided by the ICMR, India.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chibber, S., Farhan, M., Hassan, I. et al. Novel aspect of chemophototherapy in treatment of cancer. Tumor Biol. 33, 701–706 (2012). https://doi.org/10.1007/s13277-011-0288-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-011-0288-9