Abstract
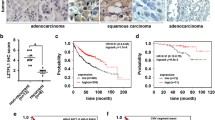
Genes, active during normal development, are frequently reactivated during neoplastic transformation and may be related to progression. One of them, the transcription factor TP63, is crucial for pulmonary epithelial development and a possible target of the recurrent 3q amplifications in lung squamous cell carcinoma (SCC). Here, we explored whether TP63 reactivation could be associated to cancer progression in lung SCC through an epithelial to mesenchymal transition. We studied TP63 amplification and TP63 expression at RNA and protein levels and we analyzed the ΔNTP63/TATP63 ratio that quantifies the proportion of the isoform lacking the transactivation domain/the isoform containing the transactivation domain. We correlated TP63 status to survival and to the expression of epithelial (E-cadherin and plakoglobin) and mesenchymal (N-cadherin, vimentin, TWIST1, and SNAIL) markers. We found that high ΔN/TA TP63 ratio was related to high E-cadherin and plakoglobin mRNA levels (P < 0.05) and that E-cadherin mRNA level was the only marker related to survival. Kaplan–Meier survival curves stratified according to the expression level of E-cadherin showed, as already reported in breast cancer, that patients with low (first quartile) or high (last quartile) E-cadherin expression had a worse survival with respect to patients with intermediate E-cadherin expression. Altogether, our results indicate that a reactivation of ΔNTP63 is linked to the maintenance of epithelial markers and suggest that E-cadherin has a dual role in lung SCC.



Similar content being viewed by others
References
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. Egfr mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500.
Chujo M, Noguchi T, Miura T, Arinaga M, Uchida Y, Tagawa Y. Comparative genomic hybridization analysis detected frequent overrepresentation of chromosome 3q in squamous cell carcinoma of the lung. Lung Cancer. 2002;38:23–9.
Umayahara K, Numa F, Suehiro Y, Sakata A, Nawata S, Ogata H, et al. Comparative genomic hybridization detects genetic alterations during early stages of cervical cancer progression. Genes Chromosomes Cancer. 2002;33:98–102.
Sattler HP, Lensch R, Rohde V, Zimmer E, Meese E, Bonkhoff H, et al. Novel amplification unit at chromosome 3q25–q27 in human prostate cancer. Prostate. 2000;45:207–15.
Lambros MB, Fiegler H, Jones A, Gorman P, Roylance RR, Carter NP, et al. Analysis of ovarian cancer cell lines using array-based comparative genomic hybridization. J Pathol. 2005;205:29–40.
Murata K, Ota S, Niki T, Goto A, Li CP, Ruriko UM, et al. P63—key molecule in the early phase of epithelial abnormality in idiopathic pulmonary fibrosis. Exp Mol Pathol. 2007;83:367–76.
Qian J, Massion PP. Role of chromosome 3q amplification in lung cancer. J Thorac Oncol. 2008;3:212–5.
Finlan LE, Hupp TR. P63: the phantom of the tumor suppressor. Cell Cycle. 2007;6:1062–71.
Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. P63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16.
Massion PP, Taflan PM, Jamshedur Rahman SM, Yildiz P, Shyr Y, Edgerton ME, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res. 2003;63:7113–21.
Pelosi G, Pasini F, Olsen Stenholm C, Pastorino U, Maisonneuve P, Sonzogni A, et al. P63 immunoreactivity in lung cancer: yet another player in the development of squamous cell carcinomas? J Pathol. 2002;198:100–9.
Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2009;101:293–9.
Herfs M, Hubert P, Suarez-Carmona M, Reschner A, Saussez S, Berx G, et al. Regulation of p63 isoforms by snail and slug transcription factors in human squamous cell carcinoma. Am J Pathol. 2010;176:1941–9.
Higashikawa K, Yoneda S, Tobiume K, Taki M, Shigeishi H, Kamata N. Snail-induced down-regulation of deltanp63alpha acquires invasive phenotype of human squamous cell carcinoma. Cancer Res. 2007;67:9207–13.
Gu X, Coates PJ, Boldrup L, Nylander K. P63 contributes to cell invasion and migration in squamous cell carcinoma of the head and neck. Cancer Lett. 2008;263:26–34.
Higashikawa K, Yoneda S, Tobiume K, Saitoh M, Taki M, Mitani Y, et al. DeltaNp63alpha-dependent expression of Id-3 distinctively suppresses the invasiveness of human squamous cell carcinoma. Int J Cancer. 2009;124:2837–44.
Koster MI, Lu SL, White LD, Wang XJ, Roop DR. Reactivation of developmentally expressed p63 isoforms predisposes to tumor development and progression. Cancer Res. 2006;66:3981–6.
Lo Muzio L, Campisi G, Farina A, Rubini C, Pastore L, Giannone N, et al. Effect of p63 expression on survival in oral squamous cell carcinoma. Cancer Invest. 2007;25:464–9.
Morita M, Uramoto H, Nakata S, Ono K, Sugaya M, Yoshimatsu T, et al. Expression of deltanp63 in squamous cell carcinoma of the esophagus. Anticancer Res. 2005;25:3533–9.
UICC. International Union Against Cancer: TNM classification of malignant tumours. 6th ed. New York: Wiley; 2002.
Saviozzi S, Cordero F, Lo Iacono M, Novello S, Scagliotti GV, Calogero RA. Selection of suitable reference genes for accurate normalization of gene expression profile studies in non-small cell lung cancer. BMC Cancer. 2006;6:200.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(−delta delta c(t)) method. Methods. 2001;25:402–8.
Blons H, Pallier K, Le Corre D, Danel C, Tremblay M, Houdayer C, et al. Genome wide snp comparative analysis between egfr and kras mutated nsclc and characterization of two models of oncogenic cooperation in non-small cell lung carcinoma. BMC Med Genomics. 2008;1:25.
World Health Organization classification of tumours. Travis WD, Brambilla E, Muller-Hermlink HK, Harris CC, editors. Lyon: IARC Press; 2004.
Westfall MD, Pietenpol JA. P63: molecular complexity in development and cancer. Carcinogenesis. 2004;25:857–64.
Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembele D, et al. Sox2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One. 2010;5:e8960.
Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15:6008–17.
Psyrri A, Papageorgiou S, Liakata E, Scorilas A, Rontogianni D, Kontos CK, et al. Phosphatidylinositol 3′-kinase catalytic subunit alpha gene amplification contributes to the pathogenesis of mantle cell lymphoma. Clin Cancer Res. 2009;15:5724–32.
Lin SC, Liu CJ, Ko SY, Chang HC, Liu TY, Chang KW. Copy number amplification of 3q26–27 oncogenes in microdissected oral squamous cell carcinoma and oral brushed samples from areca chewers. J Pathol. 2005;206:417–22.
Jorda M, Gomez-Fernandez C, Garcia M, Mousavi F, Walker G, Mejias A, et al. P63 differentiates subtypes of nonsmall cell carcinomas of lung in cytologic samples: implications in treatment selection. Cancer Cytopathol. 2009;117:46–50.
Redon R, Muller D, Caulee K, Wanherdrick K, Abecassis J, du Manoir S. A simple specific pattern of chromosomal aberrations at early stages of head and neck squamous cell carcinomas: Pik3ca but not p63 gene as a likely target of 3q26-qter gains. Cancer Res. 2001;61:4122–9.
Nylander K, Coates PJ, Hall PA. Characterization of the expression pattern of p63 alpha and delta np63 alpha in benign and malignant oral epithelial lesions. Int J Cancer. 2000;87:368–72.
Parsa R, Yang A, McKeon F, Green H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J Invest Dermatol. 1999;113:1099–105.
Borrelli S, Candi E, Alotto D, Castagnoli C, Melino G, Vigano MA, et al. P63 regulates the caspase-8-flip apoptotic pathway in epidermis. Cell Death Differ. 2009;16:253–63.
Gressner O, Schilling T, Lorenz K, Schulze Schleithoff E, Koch A, Schulze-Bergkamen H, et al. Tap63alpha induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 2005;24:2458–71.
Mundt HM, Stremmel W, Melino G, Krammer PH, Schilling T, Muller M. Dominant negative (DeltaN) p63alpha induces drug resistance in hepatocellular carcinoma by interference with apoptosis signaling pathways. Biochem Biophys Res Commun. 2010;396:335–41.
Harmes DC, Bresnick E, Lubin EA, Watson JK, Heim KE, Curtin JC, et al. Positive and negative regulation of deltan-p63 promoter activity by p53 and deltan-p63-alpha contributes to differential regulation of p53 target genes. Oncogene. 2003;22:7607–16.
Lena AM, Shalom-Feuerstein R, di Val R, Cervo P, Aberdam D, Knight RA, et al. Mir-203 represses ‘stemness’ by repressing deltanp63. Cell Death Differ. 2008;15:1187–95.
Craig AL, Holcakova J, Finlan LE, Nekulova M, Hrstka R, Gueven N, et al. DeltaNp63 transcriptionally regulates atm to control p53 serine-15 phosphorylation. Mol Cancer. 2010;9:195.
Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–13.
Kommagani R, Leonard MK, Lewis S, Romano RA, Sinha S, Kadakia MP. Regulation of vdr by deltanp63alpha is associated with inhibition of cell invasion. J Cell Sci. 2009;122:2828–35.
Urist MJ, Di Como CJ, Lu ML, Charytonowicz E, Verbel D, Crum CP, et al. Loss of p63 expression is associated with tumor progression in bladder cancer. Am J Pathol. 2002;161:1199–206.
Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. P63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–31.
Maddau C, Confortini M, Bisanzi S, Janni A, Montinaro F, Paci E, et al. Prognostic significance of p53 and ki-67 antigen expression in surgically treated non-small cell lung cancer: immunocytochemical detection with imprint cytology. Am J Clin Pathol. 2006;125:425–31.
Iwata T, Uramoto H, Sugio K, Fujino Y, Oyama T, Nakata S, et al. A lack of prognostic significance regarding deltanp63 immunoreactivity in lung cancer. Lung Cancer. 2005;50:67–73.
Gu J, Berman D, Lu C, Wistuba II, Roth JA, Frazier M, et al. Aberrant promoter methylation profile and association with survival in patients with non-small cell lung cancer. Clin Cancer Res. 2006;12:7329–38.
Mauro LJ, Wenzel SJ, Sindberg GM. Regulation of chick bone growth by leptin and catecholamines. Poult Sci. 2010;89:697–708.
Querzoli P, Coradini D, Pedriali M, Boracchi P, Ambrogi F, Raimondi E, et al. An immunohistochemically positive E-cadherin status is not always predictive for a good prognosis in human breast cancer. Br J Cancer. 2010;103:1835–9.
Miao Y, Liu N, Zhang Y, Liu Y, Yu JH, Dai SD, et al. P120ctn isoform 1 expression significantly correlates with abnormal expression of E-cadherin and poor survival of lung cancer patients. Med Oncol. 2010;27:880–6.
Kase S, Sugio K, Yamazaki K, Okamoto T, Yano T, Sugimachi K. Expression of e-cadherin and beta-catenin in human non-small cell lung cancer and the clinical significance. Clin Cancer Res. 2000;6:4789–96.
Acknowledgments
This work is part of the national program Cartes d’Identité des Tumeurs ® (CIT) http://cit.ligue-cancer.net/ which is funded and developed by the Ligue Nationale Contre le Cancer. Grant support was provided by INCA Institute (Institut du Cancer), grant 09004CP.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(XLS 24 kb)
Supplementary Table 2
Relative expression of CDH1, JUP, DN/TA TP63 ratio, CDH2, VIM, and TWIST according to non-tumor lung tissue in each SCC sample. (DOC 164 kb)
Rights and permissions
About this article
Cite this article
Pallier, K., Cazes, A., El Khattabi, L. et al. DeltaN TP63 reactivation, epithelial phenotype maintenance, and survival in lung squamous cell carcinoma. Tumor Biol. 33, 41–51 (2012). https://doi.org/10.1007/s13277-011-0239-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-011-0239-5