Abstract
Background
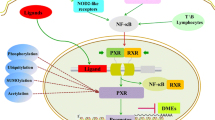
Nuclear receptor are major regulators of hepatic drug metabolizing enzymes and antioxidants enzymes. Nuclear receptor humanized mice were used for overcome species differences between experimental animals and human.
Objective
The present study was performed to investigate the hepatic regulation of antioxidant enzymes in pregnane X receptor (PXR) and constitutive androstane receptor (CAR) double humanized mice treated with the human PXR ligand, rifampicin (RIF; 10 mg/kg for 4 days).
Results
RIF decreased the hepatic protein levels of superoxide dismutase-1, thioredoxin-1, and γ-glutamylcysteine ligase catalytic subunit in wild-type (WT) mice, but not in the double humanized mice. Catalase protein levels were decreased by RIF in both WT and double humanized mice. The hepatic protein level and activity of glutathione reductase (GR) were increased in the humanized mice treated with RIF, but decreased in WT mice. Glutathione S-transferase (GST) alpha-class (GSTA) and mu-class (GSTM) but not pi-class were induced by RIF in the humanized mice, but not in WT mice. The activities of total GST, GSTA and GSTM were also increased only in humanized mice treated with RIF.
Conclusion
These results suggest that PXR and CAR may play roles in xenobiotic-induced hepatic regulation of GSTA, GSTM, and GR. The PXR/CAR double humanized mouse can be used as a suitable predictive model of the regulation of human antioxidant enzymes by xenobiotics.




Similar content being viewed by others
References
Arakawa S et al (2012) Evaluation of hepatic glutathione transferase Mu 1 and Theta 1 activities in humans and mice using genotype information. Drug Metab Dispos 40(3):497–503. https://doi.org/10.1124/dmd.111.042911 (PMID: 8074309)
Cheng X, Klaassen CD (2006) Regulation of mRNA expression of xenobiotic transporters by the pregnane x receptor in mouse liver, kidney and intestine. Drug Metab Dispos 34(11):1863–1867. https://doi.org/10.1124/dmd.106.010520 (PMID: 16928788)
Cherian MT, Chai SC, Chen T (2015) Small-molecule modulators of the constitutive androstane receptor. Expert Opin Drug Metab Toxicol 11(7):1099–1114. https://doi.org/10.1517/17425255.2015.1043887 (PMID: 25979168)
Dekeyser JG, and Omiecinski CJ (2010) Constitutive androstane receptor. In: McQueen CA (ed) Comprehensive Toxicology: Vol. 2-Cellular and Molecular Toxicology, 2nd ed., New York, Elsevier, pp 169–181
Falkner KC, Pinaire JA, Xiao GH, Geoghegan TE, Prough RA (2001) Regulation of the rat glutathione S-transferase A2 gene by glucocorticoids: involvement of both the glucocorticoid and pregnane X receptors. Mol Pharmacol 60(3):611–619 (PMID:11502894)
Gong H et al (2006) Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol 20(2):279–290. https://doi.org/10.1210/me.2005-0205 (PMID:16195250)
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405. https://doi.org/10.1016/s0076-6879(81)77053-8 (PMID: 7329316)
Habig WH et al (1974) The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci USA 71:3879–3882. https://doi.org/10.1073/pnas.71.10.3879 (PMID: 4139704)
Harvey CJ et al (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46(4):443–453. https://doi.org/10.1016/j.freeradbiomed.2008.10.040 (PMID: 19028565)
Hayes JD, Pulford DJ (1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30:445–600. https://doi.org/10.3109/10409239509083491 (PMID: 8770536)
Higgins LG, Hayes JD (2011) Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev 43(2):92–137. https://doi.org/10.3109/03602532.2011.567391 (PMID: 21495793)
Ihunnah CA, Jiang M, Xie W (2011) Nuclear receptor PXR, transcriptional circuits and metabolic relevance. Biochim Biophys Acta 1812(8):956–963. https://doi.org/10.1016/j.bbadis.2011.01.014 (PMID: 21295138)
Kandel BA et al (2016) Genomewide comparison of the inducible transcriptomes of nuclear receptors CAR, PXR and PPARα in primary human hepatocytes. Biochim Biophys Acta 1859(9):1218–1227. https://doi.org/10.1016/j.bbagrm.2016.03.007 (PMID:26994748)
Kiyosawa N et al (2008) Species-specific regulation of PXR/CAR/ER-target genes in the mouse and rat liver elicited by o, p’-DDT. BMC Genomics 9:487. https://doi.org/10.1186/1471-2164-9-487 (PMID:18925944)
Knight TR, Choudhuri S, Klaassen CD (2008) Induction of hepatic glutathione S-transferases in male mice by prototypes of various classes of microsomal enzyme inducers. Toxicol Sci 106(2):329–338. https://doi.org/10.1093/toxsci/kfn179 (PMID: 18723825)
Lee SY et al (2015) Expression of hepatic cytochrome P450s and UDP-glucuronosyltransferases in PXR and CAR double humanized mice treated with rifampicin. Toxicol Lett 235(2):107–115. https://doi.org/10.1016/j.toxlet.2015.03.015 (PMID:25835148)
Lin W et al (2020) CITCO directly binds to and activates human pregnane X receptor. Mol Pharmacol 97(3):180–190. https://doi.org/10.1124/mol.119.118513 (PMID: 31882411)
Ma X et al (2007) The PREgnane X receptor gene-humanized mouse: a model for investigating drug–drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos 35(2):194–200. https://doi.org/10.1124/dmd.106.012831 (PMID: 17093002)
Maglich JM et al (2002) Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol 62(3):638–646. https://doi.org/10.1124/mol.62.3.638 (PMID: 12181440)
Naspinski C et al (2008) Pregnane X receptor protects HepG2 cells from BaP-induced DNA damage. Toxicol Sci 104(1):67–73. https://doi.org/10.1093/toxsci/kfn058 (PMID: 18381355)
Omiecinski CJ et al (2011) Multi-species analyses of direct activators of the constitutive androstane receptor. Toxicol Sci 123(2):550–562. https://doi.org/10.1093/toxsci/kfr191 (PMID: 21778469)
Oswal DP et al (2013) Divergence between human and murine peroxisome proliferator-activated receptor alpha ligand specificities. J Lipid Res 54(9):2354–2365. https://doi.org/10.1194/jlr.M035436 (PMID: 23797899)
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Phillips MF, Mantle TJ (1993) Inactivation of mouse liver glutathione S-transferase YfYf (Pi class) by ethacrynic acid and 5,5′-dithiobis-(2-nitrobenzoic acid). Biochem J 294(Pt 1):57–62. https://doi.org/10.1042/bj2940057 (PMID: 8363586)
Pinkus R, Weiner LM, Daniel V (1996) Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J Biol Chem 271(23):13422–13429. https://doi.org/10.1074/jbc.271.23.13422 (PMID:8662787)
Qatanani M, Moore DD (2005) CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab 6(4):329–339. https://doi.org/10.2174/1389200054633899 (PMID: 16101572)
Ricci G et al (1994) Colorimetric and fluorometric assays of glutathione transferase based on 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole. Anal Biochem 218:463–465. https://doi.org/10.1006/abio.1994.1209 (PMID: 8074309)
Ryu CS et al (2014) Expression of hepatic antioxidant enzymes in non-obese type-2 diabetic Goto-Kakizaki rats. Arch Pharm Res 37(10):1345–1353. https://doi.org/10.1007/s12272-013-0267-3 (PMID: 24254933)
Scheer N et al (2008) A novel panel of mouse models to evaluate the role of human pregnane X receptor and constitutive androstane receptor in drug response. J Clin Invest 118(9):3228–3239. https://doi.org/10.1172/jci35483 (PMID: 18677425)
Schnekenburger M, Karius T, Diederich M (2014) Regulation of epigenetic traits of the glutathione S-transferase P1 gene: from detoxification toward cancer prevention and diagnosis. Front Pharmacol 5:170. https://doi.org/10.3389/fphar.2014.00170 (PMID: 25076909)
Shen G, Kong AN (2009) Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm Drug Dispos 30(7):345–355. https://doi.org/10.1002/bdd.680 (PMID:19725016)
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5’-dithiobis (2-nitrobenzoic acid). Anal Biochem 175(2):408–413. https://doi.org/10.1016/0003-2697(88)90564-7
Sonoda J et al (2005) Pregnane X receptor prevents hepatorenal toxicity from cholesterol metabolites. Proc Natl Acad Sci USA 102(6):2198–2203. https://doi.org/10.1073/pnas.0409481102 (PMID:15671183)
Stedman CA et al (2005) Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA 102(6):2063–2068. https://doi.org/10.1073/pnas.0409794102 (PMID: 15684063)
Swales KE et al (2012) Pregnane X receptor regulates drug metabolism and transport in the vasculature and protects from oxidative stress. Cardiovasc Res 93(4):674–681. https://doi.org/10.1093/cvr/cvr330 (PMID:22166712)
Szarka CE et al (1995) Glutathione S-transferase activity and glutathione S-transferase mu expression in subjects with risk for colorectal cancer. Cancer Res 55(13):2789–2793 (PMID: 7796404)
Talalay P (2000) Chemoprotection against cancer by induction of phase 2 enzymes. BioFactors 12(1–4):5–11. https://doi.org/10.1002/biof.5520120102 (PMID: 11216505)
Tirona RG, Leake BF, Podust LM, Kim RB (2004) Identification of amino acids in rat pregnane X receptor that determine species-specific activation. Mol Pharmacol 65(1):36–44. https://doi.org/10.1124/mol.65.1.36 (PMID: 14722235)
Vogel HG, Maas J, Hock FJ, Mayer D (2006) Drug discovery and evaluation: safety and pharmacokinetic assays. In: Vogel HG (ed) With 125 tables. Springer Science & Business Media, New York, pp 1–889
Wang Z et al (2010) Decreased expression of GST pi is correlated with a poor prognosis in human esophageal squamous carcinoma. BMC Cancer 10:352. https://doi.org/10.1186/1471-2407-10-352 (PMID: 20602752)
Wang YM, Ong SS, Chai SC, Chen T (2012) Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol 8(7):803–817. https://doi.org/10.1517/17425255.2012.685237 (PMID: 22554043)
Xie W et al (2000) Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406(6794):435–439. https://doi.org/10.1038/35019116 (PMID: 10935643)
Zhao Y, Zhang K, Giesy JP, Hu J (2015) Families of nuclear receptors in vertebrate models: characteristic and comparative toxicological perspective. Sci Rep 25(5):8554. https://doi.org/10.1038/srep08554 (PMID: 25711679)
Acknowledgements
This research was supported by a National Research Foundation of Korea Grant funded by the Korean Government (2018M3A9H1021640 and 2021R1A2C2004696) and Korea Institute of Science and Technology Europe basic research program (Project no. 12001).
Author information
Authors and Affiliations
Contributions
YJC, CSR, SYL, SJO and SKK conceptualized and design experiments. YJC, CSR, SYL, HGK, NYK, JYL, HJP, SWC, JHK performed the experiments and analyzed data. YJC, CSR, SYL and SKK wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Young Jae Choi has declared that no competing interests exist. Chang Seon Ryu has declared that no competing interests exist. Sang Yoon Lee has declared that no competing interests exist. Ha Gyeong Kim has declared that no competing interests exist. Nan Young Kim has declared that no competing interests exist. Ji-Yoon Lee has declared that no competing interests exist. Soo Jin Oh has declared that no competing interests exist. Han-Jin Park has declared that no competing interests exist. Seung-Woo Cho has declared that no competing interests exist. Jong-Hoon Kim has declared that no competing interests exist. Sang Kyum Kim has declared that no competing interests exist.
Ethical approval
All experimental protocols and procedures were approved and conducted according to the approved guidelines and regulations of the Institutional Animal Care and Use Committee (IACUC) of the Korea Research Institute of Bioscience and Biotechnology (KRIBB).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, Y.J., Ryu, C.S., Lee, S.Y. et al. Effects of rifampicin on hepatic antioxidant enzymes in PXR and CAR double humanized mice. Mol. Cell. Toxicol. 17, 277–286 (2021). https://doi.org/10.1007/s13273-021-00134-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-021-00134-9