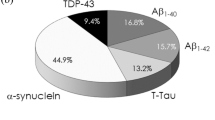
Abstract
Background
Lewy bodies are pathological hallmarks for the diagnosis of Parkinson’s disease, where the core components are composed of aggregated forms of α-synuclein (α-syn). Although α-syn has been investigated as a potential biomarker for PD, its usage has been limited and still remains controversial.
Objective
An accurate enzyme-linked immunosorbent assay (ELISA) was developed for the detection of α-syn in plasma. The hemolysis score was calculated to eliminate the additive α-syn levels from RBCs. Human plasma samples were collected in heparinized blood tubes from idiopathic Parkinson disease (IPD) and healthy control (HC).
Result
Hemolysis score had a strong correlation with the level of plasma α-syn. From the limited set of samples in this preliminary study, decreased α-syn concentrations were observed in patients with IPD in comparison to HC after adjusting for hemolysis factor. Similar results with a commercial ELISA kit were found for measuring α-syn from the same set of samples with lower correlation and the reduced accuracy of diagnosis than the current study.
Conclusion
The adjustments for the hemolysis factor would be indispensable, supporting plasma α-syn as a potential surrogate biomarker for distinguishing IPD from HC.




Similar content being viewed by others
References
Appierto V et al (2014) A lipemia-independent NanoDrop®-based score to identify hemolysis in plasma and serum samples. Bioanalysis 6:1215–1226
Barbour R et al (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5:55–59
Bartels T, Choi JG, Selkoe DJ (2011) α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477:107
Branco DM et al (2010) Cross-talk between mitochondria and proteasome in Parkinson's disease pathogenesis. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2010.0001
Buddhala C, Campbell MC, Perlmutter JS, Kotzbauer PT (2015) Correlation between decreased CSF α-synuclein and Aβ1–42 in Parkinson disease. Neurobiol Aging 36:476–484
Campbell BC et al (2000) Accumulation of insoluble alpha-synuclein in dementia with Lewy bodies. Neurobiol Dis 7:192–200
Culvenor JG et al (1999) Non-Abeta component of Alzheimer's disease amyloid (NAC) revisited. NAC and alpha-synuclein are not associated with Abeta amyloid. Am J Pathol 155:1173–1181
Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D (2013) In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells. J Biol Chem 288:6371–6385
Dettmer U et al (2015) Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun 6:7314
Dickson DW et al (1999) Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol 155:1241–1251
El-Agnaf OM et al (2006) Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J 20:419–425
Foulds PG et al (2011) Phosphorylated alpha-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson's disease. FASEB J 25:4127–4137
Foulds PG et al (2012) Post mortem cerebrospinal fluid α-synuclein levels are raised in multiple system atrophy and distinguish this from the other α-synucleinopathies, Parkinson's disease and Dementia with Lewy bodies. Neurobiol Dis 45:188–195
Foulds PG et al (2013) A longitudinal study on α-synuclein in blood plasma as a biomarker for Parkinson's disease. Sci Rep 3:2540
Gorostidi A et al (2012) α-Synuclein levels in blood plasma from LRRK2 mutation carriers. PLoS ONE 7:e52312
Gould N et al (2014) Evidence of native α-synuclein conformers in the human brain. J Biol Chem 289:7929–7934
Hall S, Öhrfelt A, Constantinescu R et al (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 69:1445–1452
Hong Z et al (2010) DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain 133:713–726
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Irwin DJ, Lee VM, Trojanowski JQ (2013) Parkinson's disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci 14:626–636
Ishii R et al (2015) Decrease in plasma levels of α-synuclein is evident in patients with Parkinson’s disease after elimination of heterophilic antibody interference. PLoS ONE 10:e0123162
Kalia LV, Lang AE (2015) Parkinson's disease. Lancet 386:896–912
Kang J, Irwin DJ, Chen-Plotkin AS et al (2013) Association of cerebrospinal fluid β-amyloid 1–42, t-tau, p-tau181, and α-synuclein levels with clinical features of drug-naive patients with early parkinson disease. JAMA Neurol 70:1277–1287
Kang M, Kim SY, An SSA, Ju YR (2013) Characterizing affinity epitopes between prion protein and β-amyloid using an epitope mapping immunoassay. Exp Mol Med 45:e34
Klucken J et al (2006) Clinical and biochemical correlates of insoluble alpha-synuclein in dementia with Lewy bodies. Acta Neuropathol 111:101–108
Kuusisto E, Parkkinen L, Alafuzoff I (2003) Morphogenesis of Lewy bodies: dissimilar incorporation of alpha-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol 62:1241–1253
Lebouvier T et al (2010) Biopsable neural tissues: toward new biomarkers for Parkinson’s disease? Front Psychiatry 1:128
Lee PH et al (2006) The plasma alpha-synuclein levels in patients with Parkinson's disease and multiple system atrophy. J Neural Transm 113:1435–1439
Lees AJ, Hardy J, Revesz T (2009) Parkinson's disease. The Lancet 373:2055–2066
Li QX et al (2007) Plasma alpha-synuclein is decreased in subjects with Parkinson's disease. Exp Neurol 204:583–588
Lin C-H et al (2017) Plasma α-synuclein predicts cognitive decline in Parkinson’s disease. J Neurol Neurosurge Psychiatry 88:818–824
Litvan I et al (1998) Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol 55:969–978
Luth ES, Bartels T, Dettmer U, Kim NC, Selkoe DJ (2015) Purification of α-synuclein from human brain reveals an instability of endogenous multimers as the protein approaches purity. Biochemistry 54:279–292
Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K (2003) The role of α-synuclein in Parkinson's disease: insights from animal models. Nat Rev Neurosci 4:727
McCann H, Stevens CH, Cartwright H, Halliday GM (2014) α-Synucleinopathy phenotypes. Parkinsonism Relat Dis 20:S62–S67
Mollenhauer B et al (2008) Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol 213:315–325
Mollenhauer B et al (2011) alpha-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 10:230–240
Ohrfelt A et al (2009) Cerebrospinal fluid alpha-synuclein in neurodegenerative disorders-a marker of synapse loss? Neurosci Lett 450:332–335
Pals P et al (2004) alpha-Synuclein promoter confers susceptibility to Parkinson's disease. Ann Neurol 56:591–595
Park MJ, Cheon SM, Bae HR, Kim SH, Kim JW (2011) Elevated levels of alpha-synuclein oligomer in the cerebrospinal fluid of drug-naive patients with Parkinson's disease. J Clin Neurol 7:215–222
Parnetti L et al (2011) Cerebrospinal fluid Tau/alpha-synuclein ratio in Parkinson's disease and degenerative dementias. Mov Disord 26:1428–1435
Parnetti L et al (2014) Differential role of CSF alpha-synuclein species, tau, and Aβ42 in Parkinson's disease. Front Aging Neurosci 6:53
Parnetti L, Cicognola C, Eusebi P, Chiasserini D (2016) Value of cerebrospinal fluid α-synuclein species as biomarker in Parkinson's diagnosis and prognosis. Biomark Med 10:35–49
Postuma RB et al (2015) MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 30:1591–1601
Rajput AH, Rozdilsky B, Rajput A (1991) Accuracy of clinical diagnosis in parkinsonism–a prospective study. Can J Neurol Sci 18:275–278
Spillantini MG et al (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840
Sui Y-T, Bullock KM, Erickson MA, Zhang J, Banks WA (2014) Alpha synuclein is transported into and out of the brain by the blood–brain barrier. Peptides 62:197–202
Tokuda T et al (2006) Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's disease. Biochem Biophys Res Commun 349:162–166
Tokuda T et al (2010) Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75:1766–1772
Tolosa E, Wenning G, Poewe W (2006) The diagnosis of Parkinson's disease. Lancet Neurol 5:75–86
Van Giau V, An SSA (2019) Epitope mapping immunoassay analysis of the interaction between beta-amyloid and fibrinogen. Int J Mol Sci 20:496
Venda LL, Cragg SJ, Buchman VL, Wade-Martins R (2010) α-Synuclein and dopamine at the crossroads of Parkinson's disease. Trends Neurosci 33:559–568
Wakabayashi K, Tanji K, Mori F, Takahashi H (2007) The Lewy body in Parkinson's disease: Molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology 27:494–506
Wang H et al (2012) Cerebrospinal fluid α-synuclein levels are elevated in multiple sclerosis and neuromyelitis optica patients during replase. J Neurochem 122:19–23
Acknowledgements
This research was supported by a National Research Foundation of Korea (NRF) Grants awarded by the Korean Government (NRF-2020R1A2B5B01002463) and Gachon University Research Fund (2018-0682). The authors would like to express their gratitude to the participants for providing the samples and their agreement to this research.
Author information
Authors and Affiliations
Contributions
Conceptualization, SSAA; investigation, KHS, SCK and Y-HS; methodology, SSAA; project administration, Y-HS and SSAA; resources, YCY and Y-HS; supervision, SSAA; validation, Y-HS and SSAA; writing—original draft, KHS; writing—review and editing, SCK, Y-HS and SSAA.
Corresponding authors
Ethics declarations
Conflict of interest
KyuHwan Shim, Seung Chan Kim, Young Chul Youn, Young-Hee Sung and Seong Soo A. An declare that they have no conflict of interest.
Human and animal rights
All patients provided written informed consent and the study was approved by the Institutional Review Board of Gachon University Gil Medical Center.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shim, K.H., Kim, S.C., Youn, Y.C. et al. Decreased plasma α-synuclein in idiopathic Parkinson’s disease patients after adjusting hemolysis factor. Mol. Cell. Toxicol. 16, 477–484 (2020). https://doi.org/10.1007/s13273-020-00104-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-020-00104-7