Abstract
Backgrounds
TOR and autophagy are essential pathways to mediate anabolic and catabolic reactions, respectively, in response to various nutritional stimuli. Vertebrate development requires such reactions to achieve the common goal of generating an independent organism from a single fertilized egg.
Methods
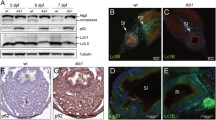
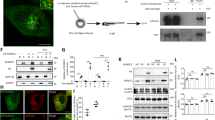
Using the zebrafish as an animal model, we characterized the role of Wdr24, a component of the GATOR2 complex that reportedly activates TORC1.
Results
Sequence analysis and subcellular localization of zebrafish Wdr24 suggested functional resemblance to its mammalian counterpart. We found that wdr24 expression commences during early embryogenesis, implicating its requirement. Accordingly, wdr24 knockdown induced defective embryogenesis accompanied by massive cell death. The developmental defects induced by wdr24 knockdown were attributable, at least in part, to dysregulated autophagy, which could be partially restored by wdr24 overexpression.
Conclusion
These findings suggest that a conserved role of Wdr24 may be a critical part of the cellular metabolism in different species.
Similar content being viewed by others
References
He, C., Bartholomew, C. R., Zhou, W. & Klionsky, D. J. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy 5, 520–526 (2009).
Boglev, Y. et al. Autophagy induction is a Tor-and Tp53-independent cell survival response in a zebrafish model of disrupted ribosome biogenesis. PLoS Genet 9, e1003279, doi:10.1371/journal.pgen.1003279 (2013).
Skobo, T. et al. Zebrafish ambra1a and ambra1b knockdown impairs skeletal muscle development. PLoS One 9, e99210, doi:10.1371/journal.pone.0099210 (2014).
Saxton, R. A. & Sabatini, D. M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976, doi:10.1016/j.cell.2017.02.004 (2017).
Laplante, M. et al. DEPTOR cell-autonomously promotes adipogenesis, and its expression is associated with obesity. Cell Metab 16, 202–212, doi:10.1016/j.cmet. 2012.07.008 (2012).
Bar-Peled, L. et al. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340, 1100–1106, doi:10.1126/science.1232044 (2013).
Panchaud, N., Peli-Gulli, M. P. & De Virgilio, C. SEACing the GAP that nEGOCiates TORC1 activation: evolutionary conservation of Rag GTPase regulation. Cell Cycle 12, 2948–2952, doi:10.4161/cc.26000 (2013).
Chantranupong, L. et al. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 9, 1–8, doi: 10.1016/j.celrep.2014.09.014 (2014).
Wei, Y. et al. TORC1 regulators Iml1/GATOR1 and GATOR2 control meiotic entry and oocyte development in Drosophila. Proc Natl Acad Sci U S A 111, E5670–5677, doi:10.1073/pnas.1419156112 (2014).
Platani, M., Trinkle-Mulcahy, L., Porter, M., Jeyaprakash, A. A. & Earnshaw, W. C. Mio depletion links mTOR regulation to Aurora A and Plk1 activation at mitotic centrosomes. J Cell Biol 210, 45–62, doi:10.1083/jcb. 201410001 (2015).
Wei, Y., Reveal, B., Cai, W. & Lilly, M. A. The GATOR1 Complex Regulates Metabolic Homeostasis and the Response to Nutrient Stress in Drosophila melanogaster. G3 (Bethesda) 6, 3859–3867, doi:10.1534/g3.116.035337 (2016).
Cai, W., Wei, Y., Jarnik, M., Reich, J. & Lilly, M. A. The GATOR2 Component Wdr24 Regulates TORC1 Activity and Lysosome Function. PLoS Genet 12, e1006036, doi:10.1371/journal.pgen.1006036 (2016).
Iida, T. & Lilly, M. A. missing oocyte encodes a highly conserved nuclear protein required for the maintenance of the meiotic cycle and oocyte identity in Drosophila. Development 131, 1029–1039, doi:10.1242/dev.01001 (2004).
Senger, S. et al. The nucleoporin Seh1 forms a complex with Mio and serves an essential tissue-specific function in Drosophila oogenesis. Development 138, 2133–2142, doi:10.1242/dev.057372 (2011).
Robu, M. E. et al. p53 activation by knockdown technologies. PLoS Genet 3, e78, doi:10.1371/journal.pgen.0030078 (2007).
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741, doi:10.1016/j.cell. 2011.10.026 (2011).
Yang, P. & Zhang, H. You are what you eat: multifaceted functions of autophagy during C. elegans development. Cell Res 24, 80–91, doi:10.1038/cr.2013.154 (2014).
McPhee, C. K. & Baehrecke, E. H. Autophagy in Drosophila melanogaster. Biochim Biophys Acta 1793, 1452–1460, doi:10.1016/j.bbamcr.2009.02.009 (2009).
Behrends, C., Sowa, M. E., Gygi, S. P. & Harper, J. W. Network organization of the human autophagy system. Nature 466, 68–76, doi:10.1038/nature09204 (2010).
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. & Schilling, T. F. Stages of embryonic development of the zebrafish. Dev Dyn 203, 253–310, doi:10.1002/aja.1002030302 (1995).
Kim, Y. I. et al. Developmental roles of D-bifunctional protein-A zebrafish model of peroxisome dysfunction. Mol Cells 37, 74–80, doi:10.14348/molcells.2014.2300 (2014).
Kim, D. et al. Fis1 depletion in osteoarthritis impairs chondrocyte survival and peroxisomal and lysosomal function. J Mol Med (Berl) 94, 1373–1384, doi:10.1007/s00109-016-1445-9 (2016).
Kim, M. J., Kang, K. H., Kim, C. H. & Choi, S. Y. Real-time imaging of mitochondria in transgenic zebrafish expressing mitochondrially targeted GFP. Biotechniques 45, 331–334, doi:10.2144/000112909 (2008).
Bhandari, S. et al. The fatty acid chain elongase, Elovl1, is required for kidney and swim bladder development during zebrafish embryogenesis. Organogenesis 12, 78–93, doi:10.1080/15476278.2016.1172164 (2016).
Kim, Y. I. et al. Cartilage development requires the function of Estrogen-related receptor alpha that directly regulates sox9 expression in zebrafish. Sci Rep 5, 18011, doi:10.1038/srep18011 (2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YI., Nam, IK., Um, JY. et al. Regulatory role of Wdr24 in autophagy activity during zebrafish embryogenesis. Mol. Cell. Toxicol. 15, 85–92 (2019). https://doi.org/10.1007/s13273-019-0010-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-019-0010-3