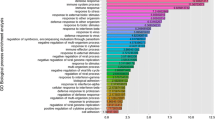
Abstract
The aim of this study is analyze the gene expression of the F2 mouse in comparison with the control mouse after exposure of F0 mouse to Di-ethyl hexyl phthalate (DEHP) via the parenteral route during the perinatal period. For this purpose, pregnant F0 mice were injected subcutaneously with corn oil (control group, n=3) or DEHP (treatment group, 30 μg/kg/day, n=3) during pregnancy and lactation. No further treatment was given after weaning of F1 mice. F1 mice were mated and delivered. Six F2 female mice from each group were randomly selected and total RNA was extracted from the ovaries. Microarrays were used to identify the genes and pathways affected by DEHP exposure. As a result, genes related with certain gynecological diseases showed significant changes in expressions. Genes, which were involved in MAPK signaling pathway, p53 signaling pathway, cell cycle, and oocyte maturation, showed significant changes in expression, too.
Similar content being viewed by others
References
Davis, B. J., Weaver, R., Gaines, L. J. & Heindel, J. J. Mono-(2-ethylhexyl) phthalate suppresses estradiol production independent of FSH-cAMP stimulation in rat granulosa cells. Toxicol Appl Pharmacol 128:224–228 (1994).
Lovekamp, T. N. & Davis, B. J. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol 172:217–224 (2001).
Davis, B. J., Maronpot, R. R. & Heindel, J. J. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol 128:216–223 (1994).
Koo, H. J. & Lee, B. M. Estimated exposure to phthalates in cosmetics and risk assessment. J Toxicol Environ Health Part A 67:1901–1914 (2004).
Guo, Y., Wang, L. & Kannan, K. Phthalates and parabens in personal care products from China: concentrations and human exposure. Arch Environ Contam Toxicol 66:113–119 (2014).
Luo, H. et al. Evaluation of the Di(2-ethylhexyl)phthalate released from polyvinyl chloride medical devices that contact blood. SpringerPlus 3:58 (2014).
Huygh, J. et al. Considerable exposure to the endocrine disrupting chemicals phthalates and bisphenol-A in intensive care unit (ICU) patients. Environ Int 81:64–72 (2015).
Singh, A. R., Lawrence, W. H. & Autian, J. Maternalfetal transfer of 14C-di-2-ethylhexyl phthalate and 14Cdiethyl phthalate in rats. J Pharm Sci 64:1347–1350 (1975).
Hopf, N. B. et al. Skin permeation and metabolism of di(2-ethylhexyl)phthalate (DEHP). Toxicol Lett 224:47–53 (2014).
Quinnies, K. M., Doyle, T. J., Kim, K. H. & Rissman, E. F. Transgenerational effects of Di-(2-Ethylhexyl) Phthalate (DEHP) on stress hormones and behavior. Endocrinol 156:3077–3083 (2015).
Manikkam, M., Tracey, R., Guerrero-Bosagna, C. & Skinner, M. K. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 8:e55387 (2013).
Cho, H. H., Kim, G. W. & Ryu, J. C. The effects of Di-2-ethylhexyl phthalates (DEHP) on the cell cycle of the endometrial cancer cell lines (ECC-1). Toxicol Environ Health Sci 6:217–223 (2014).
Doull, J. et al. A cancer risk assessment of di(2-ethylhexyl) phthalate: application of the new U.S. EPA risk assessment guidelines. Regul Toxicol Pharm 29:327–357 (1999).
Morrison, D. K. MAP kinase pathways. Cold Spring Harb Perspect Biol 4:a011254 (2012).
Zhou, B. B. & Elledge, S. J. The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439 (2000).
Bulavin, D. V. et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 18:6845–6854 (1999).
Thornton, T. M. & Rincon, M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci 5:44–51 (2009).
Jin, S. et al. Gadd45a contributes to p53 stabilization in response to DNA damage. Oncogene 22:8536–8540 (2003).
Kim, G. Y. et al. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21 (Cip1) by phosphorylation. J Biol Chem 277:29792–29802 (2002).
Zhan, Q. et al. Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene 18:2892–2900 (1999).
Hermeking, H. et al. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell 1:3–11 (1997).
Lan, C. W. et al. Functional microarray analysis of differentially expressed genes in granulosa cells from women with polycystic ovary syndrome related to MAPK/ERK signaling. Sci Rep 13;5:14994 (2015).
Diamanti-Kandarakis, E. & Dunaif. A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33:981–1030 (2012).
Wang, X., Simpson, E. R. & Brown, K. A. p53: Protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res 75:5001–5007 (2015).
Zhou, D., Zhou, C. & Chen, S. J. Gene regulation studies of aromatase expression in breast cancer and adipose stromal cells. J Steroid Biochem Mol Biol 61:273–280 (1997).
Tilly, K. I., Banerjee, S., Banerjee, P. P. & Tilly, J. L. Expression of the p53 and Wilms’ tumor suppressor genes in the rat ovary: gonadotropin repression in vivo and immunohistochemical localization of nuclear p53 protein to apoptotic granulosa cells of atretic follicles. Endocrinol 136:1394–1402 (1995).
Wang, X. X. et al. Genome-wide DNA methylation and gene expression patterns provide insight into polycystic ovary syndrome development. Oncotarget 30:6603–6610 (2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kil, K.H., Kim, M.R., Kim, J.H. et al. Analysis of ovarian gene expression in F2 mouse following perinatal exposure to DEHP via the parenteral route. Mol. Cell. Toxicol. 12, 421–427 (2016). https://doi.org/10.1007/s13273-016-0046-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-016-0046-6