Abstract
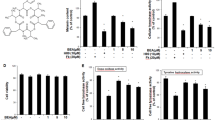
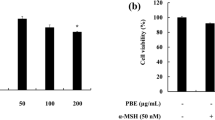
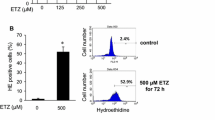
Benzo(a)pyrene (BaP) is a chemically based polycyclic aromatic hydrocarbon (PAH) that is readily absorbed by the skin. BaP is metabolized to BaP-diol-epoxide by cytochromes P-450 1A1/2 (CYP1A1/2) and cytochromes P-450 1B1 (CYP1B1) in the cytosol. BaP and its metabolites induce genotoxicity and cancer. Although BaP easily accumulates in melanin-containing tissues as well as other tissue types, the effects of BaP on melanocytes are not fully understood. Here, we show that 40-100 µM BaP represses melanin synthesis in B16F10 cells. The decrease of melanin contents is induced by tyrosinase activity in BaP-exposed B16F10. However, this repression of melanin synthesis is not induced by direct inhibition of tyrosinase in in vitro assay. Therefore, we show whether BaP regulated melanin synthesis-related enzyme. BaP regulates melanin synthesis by Tyr and Tyrpl expression. In addition, these genes expression is down-regulated by Mitf repressed by BaP. Importantly, the repression was provoked in the absence and presence of α-melanocyte stimulating hormone (α-MSH). Therefore, we hypothesize BaP interrupts the UV protection mechanism by repressing melanin synthesis in the skin. Taken together our results have revealed new side effects that exposure of BaP abolished melanin synthesis in melanocytes.
Similar content being viewed by others
References
Hodek, P. et al. The relationship between DNA adduct formation by benzo[a]pyrene and expression of its activation enzyme cytochrome P450 1A1 in rat. Environ Toxicol Pharmacol 36:989–996 (2013).
Baird, W. M., Hooven, L. A. & Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ Mol Mutagen 45:106–114 (2005).
Hamouchene, H., Arlt, V. M., Giddings, I. & Phillips, D. H. Influence of cell cycle on responses of MCF-7 cells to benzo[a]pyrene. BMC Genomics 12:333 (2011).
Phillips, D. H. & Venitt, S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int J Cancer 131:2733–2753 (2012).
Nebert, D. W., Dalton, T. P, Okey, A. B. & Gonzalez, F. J. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 279:23847–23850 (2004).
Yang, S. K., McCourt, D. W., Roller, P. P. & Gelboin, H. V. Enzymatic conversion of benzo(a)pyrene leading predominantly to the diol-epoxide r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene through a single enantiomer of r-7, t-8-dihydroxy-7,8-dihydrobenzo(a)pyrene. Proc Natl Acad Sci USA 73:2594–2598 (1976).
Kakefuda, T. & Yamamoto, H. Modification of DNA by the benzo[a]pyrene metabolite diol-epoxide r-7,t-8-dihydroxy-t-9,10-oxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci USA 75:415–419 (1978).
Straub, K. M., Meehan, T., Burlingame, A. L. & Calvin, M. Identification of the major adducts formed by reaction of benzo(a)pyrene diol epoxide with DNA in vitro. Proc Natl Acad Sci USA 74:5285–5289 (1977).
Chakravarti, D. et al. Detection of dibenzo[a,l]pyreneinduced H-ras codon 61 mutant genes in preneoplastic SENCAR mouse skin using a new PCR-RFLP method. Oncogene 16:3203–3210 (1998).
Bolotina, N. A., Gasparian, A. V., Dubovaja, T. K., Evteev, V. A. & Kobliakov, V. A. Benzo[a]pyrene-dependent activation of transcription factors NF-kappaB and AP-1 related to tumor promotion in hepatoma cell cultures. Biochemistry (Mosc) 72:552–557 (2007).
Santodonato, J., Howard, P. & Basu, D. Health and ecological assessment of polynuclear aromatic hydrocarbons. J Environ Pathol Toxicol 5:1 (1981).
Brenner, M. & Hearing, V. J. The protective role of melanin against UV damage in human skin. Photochem Photobiol 84:539–549 (2008).
Park, H. Y., Kosmadaki, M., Yaar, M. & Gilchrest, B. A. Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci 66:1493–1506 (2009).
Videira, I. F., Moura, D. F. & Magina, S. Mechanisms regulating melanogenesis. An Bras Dermatol 88:76–83 (2013).
Brenner, M. & Hearing, V. J. The protective role of melanin against UV damage in human skin. Photochem Photobiol 84:539–549 (2008).
Roberto, A., Larsson, B. S. & Tjälve, H. Uptake of 7,12-dimethylbenz(a)anthracene and benzo(a)pyrene in melanin-containing tissues. Pharmacol Toxicol 79:9299 (1996).
Lindquist, N. G. Accumulation of drugs on melanin. Acta Radiologica. Diagnosis (Stockholm) 325:1–92 (1973).
Kwon, K. J. et al. Asiaticoside, a component of Centella asiatica, inhibits melanogenesis in B16F10 mouse melanoma. Mol Med Rep 10:503–507 (2014).
Espin, J. C. & Wichers, H. J. Effect of captopril on mushroom tyrosinase activity in vitro. Biochim Biophys Acta 1544:289–300 (2001).
Catania, A., Gatti, S., Colombo, G. & Lipton, J. M. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev 56:1–29 (2004).
Delghandi, M. P., Johannessen, M. & Moens, U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal 17:1343–1351 (2005).
Bao, H., Vepakomma, M. & Sarkar, M. A. Benzo(a) pyrene exposure induces CYP1A1 activity and expression in human endometrial cells. J Steroid Biochem Mol Biol 81:37–45 (2002).
Chung, J. Y. et al. Abundance of aryl hydrocarbon receptor potentiates benzo[a]pyrene-induced apoptosis in Hepa1c1c7 cells via CYP1A1 activation. Toxicology 235:62–72 (2007).
Luecke, S. et al. The aryl hydrocarbon receptor (AHR), a novel regulator of human melanogenesis. Pigment Cell Melanoma Res 23:828–833 (2010).
Hoogduijn, M. J. et al. Melanin protects melanocytes and keratinocytes against H2O2-induced DNA strand breaks through its ability to bind Ca2+. Exp Cell Res 294:60–67 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joo, D.H., Cha, H.J., Kim, K. et al. Benzo(a)pyrene represses melanogenesis in B16F10 mouse melanoma cells. Mol. Cell. Toxicol. 11, 349–355 (2015). https://doi.org/10.1007/s13273-015-0035-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-015-0035-1