Abstract
Purposr
This study created 3D CFD models of the Norwood procedure for hypoplastic left heart syndrome (HLHS) using standard angiography and echocardiogram data to investigate the impact of shunt characteristics on pulmonary artery (PA) hemodynamics. Leveraging routine clinical data offers advantages such as availability and cost-effectiveness without subjecting patients to additional invasive procedures.
Methods
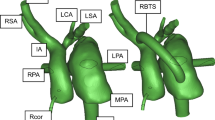
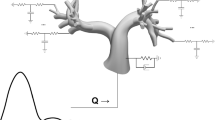
Patient-specific geometries of the intrathoracic arteries of two Norwood patients were generated from biplane cineangiograms. “Virtual surgery” was then performed to simulate the hemodynamics of alternative PA shunt configurations, including shunt type (modified Blalock-Thomas-Taussig shunt (mBTTS) vs. right ventricle-to-pulmonary artery shunt (RVPAS)), shunt diameter, and pulmonary artery anastomosis angle. Left-right pulmonary flow differential, Qp/Qs, time-averaged wall shear stress (TAWSS), and oscillatory shear index (OSI) were evaluated.
Results
There was strong agreement between clinically measured data and CFD model output throughout the patient-specific models. Geometries with a RVPAS tended toward more balanced left-right pulmonary flow, lower Qp/Qs, and greater TAWSS and OSI than models with a mBTTS. For both shunt types, larger shunts resulted in a higher Qp/Qs and higher TAWSS, with minimal effect on OSI. Low TAWSS areas correlated with regions of low flow and changing the PA-shunt anastomosis angle to face toward low TAWSS regions increased TAWSS.
Conclusion
Excellent correlation between clinically measured and CFD model data shows that 3D CFD models of HLHS Norwood can be developed using standard angiography and echocardiographic data. The CFD analysis also revealed consistent changes in PA TAWSS, flow differential, and OSI as a function of shunt characteristics.







Similar content being viewed by others
Abbreviations
- CFD:
-
computational fluid dynamics
- ESPVR:
-
end-systolic pressure-volume relationship
- HLHS:
-
hypoplastic left heart syndrome
- mBTTS:
-
modified Blalock-Thomas-Taussig shunt
- RVPAS:
-
right ventricle-to-pulmonary artery shunt
- RPA:
-
right pulmonary artery
- LPA:
-
left pulmonary artery
- PA:
-
pulmonary artery
References
Barron, D. J.; Ramchandani, B.; Murala, J.; Stumper, O.; De Giovanni, J. V.; Jones, T. J.; Stickley, J.; Brawn, W. J. Surgery Following Primary Right Ventricular Outflow Tract Stenting for Fallot’s Tetralogy and Variants: Rehabilitation of Small Pulmonary Arteries. European Journal of cardio-thoracic Surgery 2013, 44 (4), 656–662.
Bradley, S. M.; Simsic, J. M.; McQuinn, T. C.; Habib, D. M.; Shirali, G. S.; Atz, A. M. Hemodynamic Status After the Norwood Procedure: A Comparison of Right Ventricle–to–Pulmonary Artery Connection Versus Modified Blalock-Taussig Shunt. The Annals of thoracic surgery 2004, 78 (3), 933–941.
Mair, R.; Tulzer, G.; Sames, E.; Gitter, R.; Lechner, E.; Steiner, J.; Hofer, A.; Geiselseder, G.; Gross, C. Right Ventricular to Pulmonary Artery Conduit Instead of Modified Blalock-Taussig Shunt Improves Postoperative Hemodynamics in Newborns After the Norwood Operation. The Journal of Thoracic and Cardiovascular Surgery 2003, 126 (5), 1378–1384.
Pizarro, C.; Malec, E.; Maher, K. O.; Januszewska, K.; Gidding, S. S.; Murdison, K. A.; Baffa, J. M.; Norwood, W. I. Right Ventricle to Pulmonary Artery Conduit Improves Outcome After Stage I Norwood for Hypoplastic Left Heart Syndrome. Circulation 2003, 108 (10_suppl_1), II–155.
Griselli, M.; McGuirk, S. P.; Stümper, O.; Clarke, A. J.; Miller, P.; Dhillon, R.; Wright, J. G.; De Giovanni, J. V.; Barron, D. J.; Brawn, W. J. Influence of Surgical Strategies on Outcome After the Norwood Procedure. The Journal of Thoracic and cardiovascular surgery 2006, 131 (2), 418–426.
Pruetz, J. D.; Badran, S.; Dorey, F.; Starnes, V. A.; Lewis, A. B. Differential Branch Pulmonary Artery Growth After the Norwood Procedure with Right Ventricle–Pulmonary Artery Conduit Versus Modified Blalock–Taussig Shunt in Hypoplastic Left Heart Syndrome. The Journal of Thoracic and Cardiovascular Surgery 2009, 137 (6), 1342–1348.
Graham, E. M.; Atz, A. M.; Bradley, S. M.; Scheurer, M. A.; Bandisode, V. M.; Laudito, A.; Shirali, G. S. Does a Ventriculotomy Have Deleterious Effects Following Palliation in the Norwood Procedure Using a Shunt Placed from the Right Ventricle to the Pulmonary Arteries? Cardiology in the Young 2007, 17 (2), 145–150.
Newburger JW, Sleeper LA, Frommelt PC, et al. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation. 2014;129(20):2013–2020. doi:https://doi.org/10.1161/CIRCULATIONAHA.113.006191
Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362(21):1980–1992. doi:https://doi.org/10.1056/NEJMoa0912461
JW, N.; LA, S.; al., G. J. et. Transplant-Free Survival and Interventions at 6 Years in the SVR Trial. Circulation 2018, 137, 2246–2253.
Kobayashi, Y.; Kotani, Y.; Kuroko, Y.; Kawabata, T.; Sano, S.; Kasahara, S. Norwood Procedure with Right Ventricle to Pulmonary Artery Conduit: A Single-Centre 20-Year Experience. European Journal of Cardio-Thoracic Surgery 2020, 58 (2), 230–236.
Rumball, E. M.; McGuirk, S. P.; Stümper, O.; Laker, S. J.; Giovanni, J. V. de; Wright, J. G.; Barron, D. J.; Brawn, W. J. The RV–PA Conduit Stimulates Better Growth of the Pulmonary Arteries in Hypoplastic Left Heart Syndrome. European Journal of cardio-thoracic Surgery 2005, 27 (5), 801–806.
Rhodes, J.; Ubeda-Tikkanen, A.; Clair, M.; Fernandes, S. M.; Graham, D. A.; Milliren, C. E.; Daly, K. P.; Mullen, M. P.; Landzberg, M. J. Effect of Inhaled Iloprost on the Exercise Function of Fontan Patients: A Demonstration of Concept. International Journal of cardiology 2013, 168 (3), 2435–2440.
Gerrah, R.; Haller, S. J. Computational Fluid Dynamics: A Primer for Congenital Heart Disease Clinicians. Asian Cardiovascular and Thoracic Annals 2020, 28 (8), 520–532.
Bove EL, Migliavacca F, de Leval MR, et al. Use of mathematic modeling to compare and predict hemodynamic effects of the modified Blalock-Taussig and right ventricle-pulmonary artery shunts for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2008;136(2):312–320.e2. doi:https://doi.org/10.1016/j.jtcvs.2007.04.078
Mroczek T, Małota Z, Wójcik E, Nawrat Z, Skalski J. Norwood with right ventricle-to-pulmonary artery conduit is more effective than Norwood with Blalock-Taussig shunt for hypoplastic left heart syndrome: mathematic modeling of hemodynamics. Eur J Cardiothorac Surg. 2011;40(6):1412–1418. doi:https://doi.org/10.1016/j.ejcts.2011.03.033
Moghadam ME, Migliavacca F, Vignon-Clementel IE, Hsia TY, Marsden AL; Modeling of Congenital Hearts Alliance (MOCHA) Investigators. Optimization of shunt placement for the Norwood surgery using multi-domain modeling. J Biomech Eng. 2012;134(5):051002. doi:https://doi.org/10.1115/1.4006814
Piskin S, Altin HF, Yildiz O, Bakir I, Pekkan K. Hemodynamics of patient-specific aorta-pulmonary shunt configurations. J Biomech. 2017;50:166–171. doi:https://doi.org/10.1016/j.jbiomech.2016.11.014
Chen, S. J.; Carroll, J. D. 3-d Reconstruction of Coronary Arterial Tree to Optimize Angiographic Visualization. IEEE Transactions on medical imaging 2000, 19 (4), 318–336.
Sankaran, S.; Esmaily Moghadam, M.; Kahn, A. M.; Tseng, E. E.; Guccione, J. M.; Marsden, A. L. Patient-Specific Multiscale Modeling of Blood Flow for Coronary Artery Bypass Graft Surgery. Annals of biomedical engineering 2012, 40 (10), 2228–2242.
Pirola, S.; Cheng, Z.; Jarral, O.; O’Regan, D.; Pepper, J.; Athanasiou, T.; Xu, X. On the Choice of Outlet Boundary Conditions for Patient-Specific Analysis of Aortic Flow Using Computational Fluid Dynamics. Journal of Biomechanics 2017, 60, 15–21.
Sankaran, S.; Moghadam, M. E.; Kahn, A. M.; Tseng, E. E.; Guccione, J. M.; Marsden, A. L. Patient-specific multiscale modeling of blood flow for coronary artery bypass graft surgery. Annals of Biomedical Engineering 2012. https://doi.org/10.1007/s10439-012-0579-3.
Primeaux, J.; Salavitabar, A.; Lu, J. C.; Grifka, R. G.; Figueroa, C. A. Characterization of Post-Operative Hemodynamics Following the Norwood Procedure Using Population Data and Multi-Scale Modeling. Frontiers in Physiology 2021, 12, 603040.
Randles, A. P.; Kale, V.; Hammond, J.; Gropp, W.; Kaxiras, E. Performance Analysis of the Lattice Boltzmann Model Beyond Navier-Stokes. In 2013 IEEE 27th international symposium on parallel and distributed processing; IEEE, 2013; pp 1063–1074.
Randles, A.; Draeger, E. W.; Bailey, P. E. Massively Parallel Simulations of Hemodynamics in the Primary Large Arteries of the Human Vasculature. Journal of computational science 2015, 9, 70–75.
Feiger, B.; Vardhan, M.; Gounley, J.; Mortensen, M.; Nair, P.; Chaudhury, R.; Frakes, D.; Randles, A. Suitability of Lattice Boltzmann Inlet and Outlet Boundary Conditions for Simulating Flow in Image-Derived Vasculature. International journal for numerical methods in biomedical engineering 2019, 35 (6), e3198.
Pedley, T. J.; Fung, Y. The Fluid Mechanics of Large Blood Vessels. 1980.
Hsia, T. Y.; Cosentino, D.; Corsini, C.; Pennati, G.; Dubini, G.; Migliavacca, F. Use of mathematical modeling to compare and predict hemodynamic effects between hybrid and surgical norwood palliations for hypoplastic left heart syndrome. Circulation 2011, 124 (11 SUPPL. 1), 204–210. https://doi.org/10.1161/CIRCULATIONAHA.110.010769.
Ohye, R. G.; Sleeper, L. A.; Mahony, L.; Newburger, J. W.; Pearson, G. D.; Lu, M.; Goldberg, C. S.; Tabbutt, S.; Frommelt, P. C.; Ghanayem, N. S.; others. Comparison of Shunt Types in the Norwood Procedure for Single-Ventricle Lesions. New England Journal of Medicine 2010, 362 (21), 1980–1992.
Corsini, C.; Baker, C.; Kung, E.; Schievano, S.; Arbia, G.; Baretta, A.; Biglino, G.; Migliavacca, F.; Dubini, G.; Pennati, G.; others. An Integrated Approach to Patient-Specific Predictive Modeling for Single Ventricle Heart Palliation. Computer methods in biomechanics and biomedical engineering 2014, 17 (14), 1572–1589.
Raja, S. G.; Atamanyuk, I.; Tsang, V. T. Impact of Shunt Type on Growth of Pulmonary Arteries After Norwood Stage I Procedure: Current Best Available Evidence. World Journal for Pediatric and Congenital Heart Surgery 2011, 2 (1), 90–96.
Tang BT, Pickard SS, Chan FP, Tsao PS, Taylor CA, Feinstein JA. Wall shear stress is decreased in the pulmonary arteries of patients with pulmonary arterial hypertension: An image-based, computational fluid dynamics study. Pulm Circ. 2012;2(4):470–476. doi:https://doi.org/10.4103/2045-8932.105035
Chatzizisis, Y. S.; Jonas, M.; Coskun, A. U.; Beigel, R.; Stone, B. V.; Maynard, C.; Gerrity, R. G.; Daley, W.; Rogers, C.; Edelman, E. R.; Feldman, C. L.; Stone, P. H. Prediction of the localization of high-risk coronary atherosclerotic plaques based on low endothelial shear stress-an intravascular ultrasound and histopathology natural history study. Circulation 2008, 117 (8), 993–1002. https://doi.org/10.1161/CIRCULATIONAHA.107.695254.
Ene-Iordache, B.; Remuzzi, A. Blood Flow in Idealized Vascular Access for Hemodialysis: A Review of Computational Studies. Cardiovascular Engineering and Technology 2017, 8 (3), 295–312.
Tang, B. T.; Pickard, S. S.; Chan, F. P.; Tsao, P. S.; Taylor, C. A.; Feinstein, J. A. Wall Shear Stress Is Decreased in the Pulmonary Arteries of Patients with Pulmonary Arterial Hypertension: An Image-Based, Computational Fluid Dynamics Study. Pulmonary Circulation 2012, 2 (4), 470–476.
Arnaz A, Pişkin Ş, Oğuz GN, Yalçınbaş Y, Pekkan K, Sarıoğlu T. Effect of modified Blalock-Taussig shunt anastomosis angle and pulmonary artery diameter on pulmonary flow. Anatol J Cardiol. 2018;20(1):2–8. doi:https://doi.org/10.14744/AnatolJCardiol.2018.54810
Yang W, Dong M, Rabinovitch M, Chan FP, Marsden AL, Feinstein JA. Evolution of hemodynamic forces in the pulmonary tree with progressively worsening pulmonary arterial hypertension in pediatric patients. Biomech Model Mechanobiol. 2019;18(3):779–796. doi:https://doi.org/10.1007/s10237-018-011140
Vardhan, M. et al. The importance of side branches in modeling 3D hemodynamics from angiograms for patients with coronary artery disease. Sci Rep 9, 8854 (2019).
Vardhan, M. et al. Non-invasive characterization of complex coronary lesions. Sci Rep 11, 8145 (2021).
Feiger, B. et al. Suitability of lattice Boltzmann inlet and outlet boundary conditions for simulating flow in image-derived vasculature. Int J Numer Meth Biomed Engng e3198 (2019) doi:https://doi.org/10.1002/cnm.3198.
Funding
This work was supported by the Duke University Department of Medicine Eugene A. Stead Jr. Scholarship, NIH grants DP5OD019876 and DP1AG082343, the Argonne Leadership Computing Facility Aurora Early Science Program, and the Lawrence Livermore National Laboratory Institutional Computing Grand Challenge program. The content does not necessarily represent the official views of the NIH (National Institutes of Health). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as potential conflicts of interest. This work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344
Additional information
Communicated by Keefe B. Manning, PhD.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chidyagwai, S.G., Kaplan, M.S., Jensen, C.W. et al. Surgical Modulation of Pulmonary Artery Shear Stress: A Patient-Specific CFD Analysis of the Norwood Procedure. Cardiovasc Eng Tech (2024). https://doi.org/10.1007/s13239-024-00724-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13239-024-00724-3