Abstract
Purpose
Prior studies have indicated an impact of cardiac muscle viscoelasticity on systolic and diastolic functions. However, the studies of ventricular free wall viscoelasticity, particularly for that of right ventricles (RV), are limited. Moreover, investigations on ventricular passive viscoelasticity have been restricted to large animals and there is a lack of data on rodent species. To fill this knowledge gap, this study aims to develop a biaxial tester that induces high-speed physiological deformations to characterize the passive viscoelasticity of rat RVs.
Methods
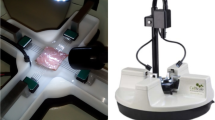
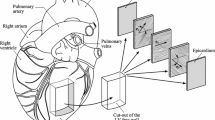
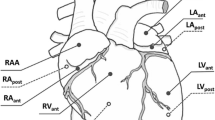
The biaxial testing system was fabricated so that planar deformation of rat ventricle tissues at physiological strain rates was possible. The testing system was validated using isotropic polydimethylsiloxane (PDMS) sheets. Next, viscoelastic measurements were performed in healthy rat RV free walls by equibiaxial cyclic sinusoidal loadings and stress relaxation.
Results
The biaxial tester’s consistency, accuracy, and stability was confirmed from the PDMS samples measurements. Moreover, significant viscoelastic alterations of the RV were found between sub-physiological (0.1 Hz) and physiological frequencies (1–8 Hz). From hysteresis loop analysis, we found as the frequency increased, the elasticity and viscosity were increased in both directions. Interestingly, the ratio of storage energy to dissipated energy (Wd/Ws) remained constant at 0.1–5 Hz. We did not observe marked differences in healthy RV viscoelasticity between longitudinal and circumferential directions.
Conclusion
This work provides a new experimental tool to quantify the passive, biaxial viscoelasticity of ventricle free walls in both small and large animals. The dynamic mechanical tests showed frequency-dependent elastic and viscous behaviors of healthy rat RVs. But the ratio of dissipated energy to stored energy was maintained between frequencies. These findings offer novel baseline information on the passive viscoelasticity of healthy RVs in adult rats.








Similar content being viewed by others
References
Yancy, C. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 128(16):1810, 2013.
Virani, S. S., and W. T. Connie. Heart disease and stroke statistics-2020 update: a report from the american heart association. Circulation. 141(9):139, 2020.
Liu, W., and Z. Wang. Current understanding of the biomechanics of ventricular tissues in heart failure. Bioengineering. 7:2, 2020. https://doi.org/10.3390/BIOENGINEERING7010002.
Kia, D. S., K. Kim, and M. A. Simon. Current understanding of the right ventricle structure and function in pulmonary arterial hypertension. Front. Physiol. 12:641310, 2021.
Caporizzo, M. A., C. Y. Chen, K. Bedi, K. B. Margulies, and B. L. Prosser. Microtubules increase diastolic stiffness in failing human cardiomyocytes and myocardium. Circulation. 2020. https://doi.org/10.1161/CIRCULATIONAHA.119.043930.
Caporizzo, M. A., C. Y. Chen, A. K. Salomon, K. B. Margulies, and B. L. Prosser. Microtubules provide a viscoelastic resistance to myocyte motion. Biophys. J. 115(9):1796–1807, 2018. https://doi.org/10.1016/j.bpj.2018.09.019.
Cooper, G. Proliferation cardiac microtubules. Heart Circul. Physiol. 297:H510, 2009.
Cooper, G. Cytoskeletal networks and the regulation of cardiac contractility: microtubules, hypertrophy, and cardiac dysfunction. Heart Circul. Physiol. 291(3):1003–1014, 2006.
Gultekin, O., G. Sommer, and G. A. Holzapfel. An orthotropic viscoelastic model for the passive myocardium: continuum basis and numerical treatment. Comput. Methods Biomech. Biomed. Eng. 19(15):1647–1664, 2016.
Dokos, S., B. H. Smaill, A. A. Young, and I. J. LeGrice. Shear properties of passive ventricular myocardium. Heart Circul. Physiol. 283(6):2650–2659, 2002.
Gerhard, S., et al. Biomechanical properties and microstructure of human ventricular myocardium. Acta Biomater. 24:172–192, 2015. https://doi.org/10.1016/J.ACTBIO.2015.06.031.
Ahmad, F., et al. Biomechanical properties and microstructure of neonatal porcine ventricles. J. Mech. Behav. Biomed. Mater. 88:18–28, 2018.
Liu, W., M. Nguyen-Truong, M. Ahern, K. M. Labus, C. M. Puttlitz, and Z. Wang. Different passive viscoelastic properties between the left and right ventricles in healthy adult ovine. J. Biomech. Eng. 2021. https://doi.org/10.1115/1.4052004.
Borgdorff, M. A. J., M. G. Dickinson, R. M. F. Berger, and B. Bartelds. Right ventricular failure due to chronic pressure load: What have we learned in animal models since the NIH working group statement? Heart Fail. Rev. 20:475–491, 2015.
Fatemifar, F., M. D. Feldman, M. Oglesby, and H. C. Han. Comparison of biomechanical properties and microstructure of trabeculae carneae, papillary muscles, and myocardium in human heart. J. Biomech. Eng. 2018. https://doi.org/10.1115/1.4041966.
Sirry, M. S., et al. Characterisation of the mechanical properties of infarcted myocardium in the rat under biaxial tension and uniaxial compression. J. Mech. Behav. Biomed. Mater. 63:252–264, 2016.
Ghaemi, H., K. Behdinan, and A. D. Spence. In vitro technique in estimation of passive mechanical properties of bovine heart: Part I. Experimental techniques and data. Med. Eng. Phys. 31(1):76–82, 2009.
Javani, S., M. Gordon, and A. N. Azadani. Biomechanical properties and microstructure of heart chambers: a paired comparison study in an ovine model. Ann. Biomed. Eng. 44(11):3266–3283, 2016. https://doi.org/10.1007/S10439-016-1658-7.
Nordsletten, D., et al. A viscoelastic model for human myocardium. Acta Biomater. 135:441–457, 2021.
Anssari-Benam, A., Y.-T. Tseng, G. A. Holzapfel, and A. Bucchi. Rate-dependency of the mechanical behaviour of semilunar heart valves under biaxial deformation. Acta Biomaterilia. 88:120–130, 2019.
Anssari-Benam, A., Y.-T. Tseng, G. A. Holzapfel, and A. Bucchi. Rate-dependent mechanical behaviour of semilunar valves under biaxial deformation: from quasi-static to physiological loading rates. J. Mech. Behav. Biomed. Mater. 104:103645, 2020.
Milani-Nejad, N., and P. M. L. Janssen. Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 141(3):235–249, 2014.
Sharp, J., T. Zammit, T. Azar, and D. Lawson. Stress-like responses to common procedures in individually and group-housed female rats. Contemp. Top. Lab Anim. Sci. 42(1):9–18, 2003.
Hill, M. R., M. A. Simon, D. Valdez-Jasso, W. Zhang, H. C. Champion, and M. S. Sacks. Structural and mechanical adaptations of right ventricle free wall myocardium to pressure overload. Ann. Biomed. Eng. 42(12):2451–2465, 2014. https://doi.org/10.1007/s10439-014-1096-3.
Jang, S., et al. Biomechanical and hemodynamic measures of right ventricular diastolic function: translating tissue biomechanics to clinical relevance. J. Am. Heart. Assoc. 2017. https://doi.org/10.1161/JAHA.117.006084.
Valdez-Jasso, D., M. A. Simon, H. C. Champion, and M. S. Sacks. A murine experimental model for the mechanical behaviour of viable right-ventricular myocardium. J. Physiol. 590:4571, 2012.
Avazmohammadi, R., M. Hill, M. Simon, and M. Sacks. Transmural remodeling of right ventricular myocardium in response to pulmonary arterial hypertension. APL Bioeng. 2017. https://doi.org/10.1063/1.5011639.
Labus, K. M., and C. M. Puttlitz. An anisotropic hyperelastic constitutive model of brain white matter in biaxial tension and structural–mechanical relationships. J. Mech. Behav. Biomed. Mater. 62:195–208, 2016.
Liu, W., et al. Multiscale contrasts between the right and left ventricle biomechanics in healthy adult sheep and translational implications. Front. Bioeng. Biotechnol. 10:588, 2022. https://doi.org/10.3389/FBIOE.2022.857638/BIBTEX.
Wang, Z., R. S. Lakes, J. C. Eickhoff, and N. C. Chesler. Effects of collagen deposition on passive and active mechanical properties of large pulmonary arteries in hypoxic pulmonary hypertension. Biomech. Model Mechanobiol. 12:1115–1125, 2013.
Gupta, K. B., M. B. Ratcliffe, M. A. Fallert, L. H. Edmunds Jr., and D. K. Bogen. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 89(5):2314–2326, 1994.
Kim, J. H., P. Chhai, and K. Rhee. Development and characterization of viscoelastic polydimethylsiloxane phantoms for simulating arterial wall motion. Med. Eng. Phys. 91:12–18, 2021.
Fung, Y. C., K. Fronek, and P. Patitucci. Pseudoelasticity of arteries and the choice of its mathematical expression. Heart Circ. Physiol. 237(5):620–631, 1979.
Caporizzo, M. A., and B. L. Prosser. Need for speed: the importance of physiological strain rates in determining myocardial stiffness. Front. Physiol. 2021. https://doi.org/10.3389/FPHYS.2021.696694.
Liu, W., et al. Alterations of biaxial viscoelastic properties of the right ventricle in pulmonary hypertension development in rest and acute stress conditions. Front. Bioeng. Biotechnol. 2023. https://doi.org/10.3389/fbioe.2023.1182703.
Liu, W., et al. Strain-dependent stress relaxation behavior of healthy right ventricular free wall. Acta Biomater. 152:290–299, 2022. https://doi.org/10.1016/J.ACTBIO.2022.08.043.
Mata, A., A. J. Fleischman, and S. Roy. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdev. 7:281–293, 2005.
Sales, F. C. P., R. M. Ariati, V. T. Noronha, and J. E. Ribeiro. Mechanical characterization of PDMS with different mixing ratios. Proc. Struct. Integr. 37:383–388, 2022.
Goyal, A., et al. In situ synthesis of metal nanoparticle embedded free standing multifunctional PDMS films. Macromol. Rapid Commun. 30:1116, 2009.
Johnston, I. D., D. K. McCluskey, C. K. L. Tan, and M. C. Tracey. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 24:035017, 2014.
Ramo, N. L., K. L. Troyer, and C. M. Puttlitz. Viscoelasticity of spinal cord and meningeal tissues. Acta. Biomaterilia. 75:253–262, 2018.
Campeau, M.-A., et al. Effect of manufacturing and experimental conditions on the mechanical and surface properties of silicone elastomer scaffolds used in endothelial mechanobiological studies. Biomed. Eng. Online. 16:90, 2017.
Chen, A. I., et al. Multilayered tissue mimicking skin and vessel phantoms with tunable mechanical, optical, and acoustic properties. Int. J. Med. Phys. Res. Pract. 43:3117, 2016.
Lakes, R. “Viscoelastic properties of materials,” Viscoelast. Mater., 2010.
Zacharatos, A., and E. Kontou. Nonlinear viscoelastic modeling of soft polymers. J. Appl. Polym. Sci. 2015. https://doi.org/10.1002/app.42141.
Hadley, D. W., and I. M. Ward. Anisotropic and nonlinear viscoelastic behaviour in solid polymers. Rep. Prog. Phys. 38(10):1143–1215, 1975.
Fehervary, H., M. Smoljkic, J. Vander Sloten, and N. Famaey. Planar biaxial testing of soft biological tissue using rakes: a critical analysis of protocol and fitting process. J. Mech. Behav. Biomed. Mater. 61:135–151, 2016.
Acknowledgements
We would like to express our gratitude to Katie Evans and Michael Nguyen-Truong for assisting in data collection, and Ethan Barron for helping in tissue bath construction.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kellan Roth, Wenqiang Liu, Kristen LeBar, Matt Ahern and Zhijie Wang declare that they have no conflict of interest.
Additional information
Associate Editor Sarah Vigmostad, Ph.D. oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roth, K., Liu, W., LeBar, K. et al. Establishment of a Biaxial Testing System for Characterization of Right Ventricle Viscoelasticity Under Physiological Loadings. Cardiovasc Eng Tech (2024). https://doi.org/10.1007/s13239-024-00722-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13239-024-00722-5