Abstract
Purpose
Tetralogy of Fallot and other conditions affecting the right ventricular outflow tract (RVOT) are common in pediatric patients, but there is a lack of quantitative comparison among techniques for repairing or replacing the pulmonary valve. The aim of this study was to develop a robust in vitro system for quantifying flow conditions after various RVOT interventions.
Methods
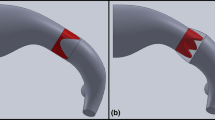
An infant-sized mock circulatory loop that includes a 3D-printed RVOT anatomical model was developed to evaluate flow conditions after different simulated surgical repairs. Physiologically correct flow and pressure were achieved with custom compliant tubing and a tunable flow restrictor. Pressure gradient, flow regurgitation, and coaptation height were measured for two monocusp leaflet designs after tuning the system with a 12 mm Hancock valved conduit.
Results
Measurements were repeatable across multiple samples of two different monocusp designs, with the wider leaflet in the 50% backwall model consistently exhibiting lower pressure gradient but higher regurgitation compared to the leaflet in the 40% backwall model. Coaptation height was measured via direct visualization with endoscopic cameras, revealing a shorter area of contact for the wider leaflet (3.3-4.0 mm) compared to the narrower one (4.3 mm).
Conclusion
The 3D-printed RVOT anatomical model and in vitro pulmonary circulatory loop developed in this work provide a platform for planning and evaluating surgical interventions in the pediatric population. Measurements of regurgitation, pressure gradient, and coaptation provide a quantitative basis for comparison among different valve designs and positions.







Similar content being viewed by others
Abbreviations
- RVOT:
-
Right-ventricular outflow tract
- ToF:
-
Tetralogy of Fallot
- MPA:
-
Main pulmonary artery
- RV:
-
Right ventricle
- e-PTFE:
-
Expanded polytetrafluoroethylene
- STJ:
-
Sinotubular junction
- BW:
-
Backwall (corresponding to native MPA tissue in transannular patch repair)
- TPU:
-
Thermoplastic polyurethane
- MCL:
-
Mock circulatory loop
- NP:
-
Normal physiology (see Table 2)
- HCO:
-
High cardiac output (see Table 2)
- HPVR:
-
High pulmonary vascular resistance (see Table 2)
- fR :
-
Regurgitant fraction
- fC :
-
Closing volume fraction
References
D. van der Linde et al, “Birth Prevalence of Congenital Heart Disease Worldwide,” J. Am. Coll. Cardiol, vol. 58, no. 21, pp. 2241–2247, Nov. 2011, doi: https://doi.org/10.1016/j.jacc.2011.08.025.
J. L. R. Romeo et al, “Outcome after surgical repair of tetralogy of Fallot: A systematic review and meta-analysis,” J. Thorac. Cardiovasc. Surg, vol. 159, no. 1, pp. 220–236.e8, Jan. 2020, doi: https://doi.org/10.1016/j.jtcvs.2019.08.127.
L. Sasson et al, “Right ventricular outflow tract strategies for repair of tetralogy of Fallot: effect of monocusp valve reconstruction,” Eur. J. Cardiothorac. Surg, vol. 43, no. 4, pp. 743–751, Apr. 2013, doi: https://doi.org/10.1093/ejcts/ezs479.
Z. Lyu, M. Jin, and Y. Yang, “Value of pulmonary annulus index in predicting transannular patch in tetralogy of Fallot repair,” J. Card. Surg, vol. 36, no. 7, pp. 2197–2203, Jul. 2021, doi: https://doi.org/10.1111/jocs.15500.
H. F. Al Habib et al, “Contemporary Patterns of Management of Tetralogy of Fallot: Data From The Society of Thoracic Surgeons Database,” Ann. Thorac. Surg, vol. 90, no. 3, pp. 813–820, Sep. 2010, doi: https://doi.org/10.1016/j.athoracsur.2010.03.110.
N. M. Singh, R. S. Loomba, T. M. Gudausky, and M. E. Mitchell, “Monocusp valve placement in children with tetralogy of Fallot undergoing repair with transannular patch: A functioning pulmonary valve does not improve immediate postsurgical outcomes,” Congenit. Heart Dis, vol. 13, no. 6, pp. 935–943, 2018, doi: https://doi.org/10.1111/chd.12670.
M. W. Turrentine, R. P. McCarthy, P. Vijay, K. W. McConnell, and J. W. Brown, “PTFE monocusp valve reconstruction of the right ventricular outflow tract,” Ann. Thorac. Surg, vol. 73, no. 3, pp. 871–880, 2002, doi: https://doi.org/10.1016/S0003-4975(01)03441-5.
E. H. N. Sayyed, S. S. Rana, K. M. Anand, A. Eram, and S. Harkant, “Monocusp pulmonary valve reconstruction in childhood and adult TOF repairs: does a single cusped valve work?,” Indian J. Thorac. Cardiovasc. Surg, vol. 32, no. 4, pp. 229–234, 2016, doi: https://doi.org/10.1007/s12055-016-0457-y.
D. S. Nath et al, “Pulmonary Homograft Monocusp Reconstruction of the Right Ventricular Outflow Tract: Outcomes to the Intermediate Term,” Ann. Thorac. Surg, vol. 90, no. 1, pp. 42–49, Jul. 2010, doi: https://doi.org/10.1016/j.athoracsur.2010.03.045.
S. R. Gundry, “Pericardial and Synthetic Monocusp Valves: Indication and Results,” Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu, vol. 2, no. 1, pp. 77–82, 1999, doi: https://doi.org/10.1016/S1092-9126(99)70007-4.
S. Pande, S. K. Agarwal, G. Majumdar, B. Chandra, P. Tewari, and S. Kumar, “Pericardial Monocusp for Pulmonary Valve Reconstruction: A New Technique,” Asian Cardiovasc. Thorac. Ann, vol. 18, no. 3, pp. 279–284, Jun. 2010, doi: https://doi.org/10.1177/0218492310369185.
J. F. M. Bechtel, P. E. Lange, and H. H. Sievers, “Optimal size of a monocusp patch for reconstruction of a hypoplastic pulmonary root: An experimental study in pigs,” Ann. Thorac. Surg, vol. 79, no. 6, pp. 2103–2108, 2005, doi: https://doi.org/10.1016/j.athoracsur.2004.11.040.
H.-H. Sievers et al, “Superior function of a bicuspid over a monocuspid patch for reconstruction of a hypoplastic pulmonary root in pigs,” J. Thorac. Cardiovasc. Surg, vol. 105, no. 4, pp. 580–590, Apr. 1993, doi: https://doi.org/10.1016/S0022-5223(19)34183-2.
S. Mosbahi et al, “Computational fluid dynamics of the right ventricular outflow tract and of the pulmonary artery: a bench model of flow dynamics,” Interact. Cardiovasc. Thorac. Surg, vol. 19, no. 4, pp. 611–616, Oct. 2014, doi: https://doi.org/10.1093/icvts/ivu202.
L. Louvelle, M. Doyle, G. Van Arsdell, and C. Amon, “The Effect of Geometric and Hemodynamic Parameters on Blood Flow Efficiency in Repaired Tetralogy of Fallot Patients,” Ann. Biomed. Eng, vol. 49, no. 9, pp. 2297–2310, 2021, doi: https://doi.org/10.1007/s10439-021-02771-6.
L. A. Vricella, S. R. Gundry, H. Izutani, M. A. Kuhn, N. Mulla, and L. L. Bailey, “Fate of polytetrafluoroethylene monocusp pulmonary valves in animal model,” Asian Cardiovasc. Thorac. Ann, vol. 11, no. 4, pp. 280–284, 2003, doi: https://doi.org/10.1177/021849230301100402.
D. Timms, M. Hayne, K. McNeil, and A. Galbraith, “A complete mock circulation loop for the evaluation of left, right, and biventricular assist devices,” Artif. Organs, vol. 29, no. 7, pp. 564–572, 2005, doi: https://doi.org/10.1111/j.1525-1594.2005.29094.x.
E. Cuenca-Navalon, T. Finocchiaro, M. Laumen, A. Fritschi, T. Schmitz-Rode, and U. Steinseifer, “Design and evaluation of a hybrid mock circulatory loop for total artificial heart testing,” Int. J. Artif. Organs, vol. 37, no. 1, pp. 71–80, 2014, doi: https://doi.org/10.5301/ijao.5000301.
D. V. Telyshev, A. A. Pugovkin, and S. V. Selishchev, “A Mock Circulatory System for Testing Pediatric Rotary Blood Pumps,” Biomed. Eng, vol. 51, no. 2, pp. 83–87, 2017, doi: https://doi.org/10.1007/s10527-017-9689-4.
I. Mueller et al, “Design of a right ventricular mock circulation loop as a test bench for right ventricular assist devices,” Biomed. Tech, vol. 62, no. 2, pp. 131–137, 2017, doi: https://doi.org/10.1515/bmt-2016-0104.
S. Shehab et al, “Valvular Regurgitation in a Biventricular Mock Circulatory Loop,” ASAIO J, vol. 65, no. 6, pp. 551–557, 2019, doi: https://doi.org/10.1097/MAT.0000000000000852.
S. D. Gregory et al, “An advanced mock circulation loop for in vitro cardiovascular device evaluation,” Artif. Organs, vol. 44, no. 6, pp. E238–E250, 2020, doi: https://doi.org/10.1111/aor.13636.
N. K. Schiavone, C. J. Elkins, D. B. McElhinney, J. K. Eaton, and A. L. Marsden, “In Vitro Assessment of Right Ventricular Outflow Tract Anatomy and Valve Orientation Effects on Bioprosthetic Pulmonary Valve Hemodynamics,” Cardiovasc. Eng. Technol, vol. 12, no. 2, pp. 215–231, 2021, doi: https://doi.org/10.1007/s13239-020-00507-6.
M. Amabili, P. Balasubramanian, G. Ferrari, G. Franchini, F. Giovanniello, and E. Tubaldi, “Identification of viscoelastic properties of Dacron aortic grafts subjected to physiological pulsatile flow,” J. Mech. Behav. Biomed. Mater, vol. 110, no. April, p. 103804, 2020, doi: https://doi.org/10.1016/j.jmbbm.2020.103804.
A. P. Yoganathan et al, “A new paradigm for obtaining marketing approval for pediatric-sized prosthetic heart valves,” J. Thorac. Cardiovasc. Surg, vol. 146, no. 4, pp. 879–886, 2013, doi: https://doi.org/10.1016/j.jtcvs.2013.04.016.
R. M. Bojar, “Cardiovascular Management,” in Manual of Perioperative Care in Adult Cardiac Surgery, 5th ed., Wiley-Blackwell, 2011, pp. 439–580.
J. Schindelin et al, “Fiji: An open-source platform for biological-image analysis,” Nat. Methods, vol. 9, no. 7, pp. 676–682, 2012, doi: https://doi.org/10.1038/nmeth.2019.
L. Mercer-Rosa, W. Yang, S. Kutty, J. Rychik, M. Fogel, and E. Goldmuntz, “Quantifying Pulmonary Regurgitation and Right Ventricular Function in Surgically Repaired Tetralogy of Fallot: A Comparative Analysis of Echocardiography and Magnetic Resonance Imaging,” Circ. Cardiovasc. Imaging, vol. 5, no. 5, pp. 637–643, Sep. 2012, doi: https://doi.org/10.1161/CIRCIMAGING.112.972588.
M. DiLorenzo, W.-T. Hwang, E. Goldmuntz, B. Ky, and L. Mercer-Rosa, “Diastolic dysfunction in tetralogy of Fallot: Comparison of echocardiography with catheterization,” Echocardiography, vol. 35, no. 10, pp. 1641–1648, Oct. 2018, doi: https://doi.org/10.1111/echo.14113.
D. J. Barron, “The Norwood Procedure: In favor of the RV-PA Conduit,” Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu, vol. 16, no. 1, pp. 52–58, Jan. 2013, doi: https://doi.org/10.1053/j.pcsu.2013.01.002.
E. M. Rumball et al, “The RV–PA conduit stimulates better growth of the pulmonary arteries in hypoplastic left heart syndrome*,” Eur. J. Cardiothorac. Surg, vol. 27, no. 5, pp. 801–806, May 2005, doi: https://doi.org/10.1016/j.ejcts.2005.01.061.
M. S. Cabrera, C. W. J. Oomens, C. V. C. Bouten, A. J. J. C. Bogers, S. P. Hoerstrup, and F. P. T. Baaijens, “Mechanical analysis of ovine and pediatric pulmonary artery for heart valve stent design,” J. Biomech, vol. 46, no. 12, pp. 2075–2081, 2013, doi: https://doi.org/10.1016/j.jbiomech.2013.04.020.
Acknowledgements
This work was supported by internal funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
S.B. Kizilski, X. Zhang, N.E. Kneier, M.D. Chaillo Lizarraga, N.E. Schulz, P.E. Hammer, and D.M. Hoganson declare that they have no conflicts of interest.
Additional information
Communicated by Igor Efimov.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kizilski, S.B., Zhang, X., Kneier, N.E. et al. An In Vitro Circulatory Loop Model of the Pediatric Right Ventricular Outflow Tract as a Platform for Valve Evaluation. Cardiovasc Eng Tech 14, 217–229 (2023). https://doi.org/10.1007/s13239-022-00648-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-022-00648-w