Abstract
Background
Myocardial tissue can be ablated by the application nanosecond pulsed fields (nsPEFs). The applied electric fields irreversibly permeabilize cell membranes and thereby kill myocytes while leaving the extracellular matrix intact.
Methods
In domestic pigs (n = 10), hearts were exposed via sternotomy and either ablated in vivo (\(n\) = 5) or in excised, Langendorff-perfused hearts (\(n\) = 5). The nsPEFs consisted of 6–36 pulses of 300 ns each, delivered at 3–6 Hz; the voltage applied varied from 10 to 12 kV. Atrial lesions were either created after inserting the bottom jaw of the bipolar clamp into the atrium via a purse string incision (2–3 lesions per atrium) or by clamping a double layer of tissue at the appendages (one lesion per atrium). Ventricular lesions were created after an incision at the apex. The transmurality of each lesion was determined at three points along the lesion using a triphenyl tetrazolium chloride (TTC) stain.
Results
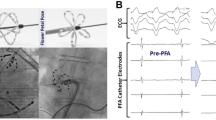
All 27 atrial lesions were transmural. This includes 13/13 purse string lesions (39/39 sections, tissue thickness 2.5–4.5 mm) and 14/14 appendage lesions (42/42 sections, tissue thickness 8–12 mm). All 3 right ventricular lesions were transmural (9/9 sections, 18 pulses per lesion). Left ventricular lesions were always transmural for 36 pulses (3/3 lesions, 9/9 sections). All lesions have highly consistent width across the wall. There were no pulse-induced arrhythmias or other complications during the procedure.
Conclusions
nsPEF ablation reliably created acute lesions in porcine atrial and ventricular myocardium. It has far better penetration and is faster than both radiofrequency ablation and cryoablation and it is free from thermal side effects.



Similar content being viewed by others
References
Ad, N., R. M. Suri, J. S. Gammie, S. Sheng, S. M. O’Brien, and L. Henry. Surgical ablation of atrial fibrillation trends and outcomes in North America. J. Thorac Cardiovasc. Surg. 144(5):1051–1060, 2012. https://doi.org/10.1016/j.jtcvs.2012.07.065
Calkins, H., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Heart Rhythm. 14(10):e445–e494, 2017. https://doi.org/10.1016/j.hrthm.2017.07.009
Cox, J. L. The first Maze procedure. J. Thorac Cardiovasc. Surg. 141(5):1093–1097, 2011. https://doi.org/10.1016/j.jtcvs.2010.12.012
Damiano, R. J., Jr., and M. Bailey. The Cox-Maze IV procedure for lone atrial fibrillation. Multimed. Man Cardiothorac. Surg. 2007(723):mmcts 2007 002758, 2007. https://doi.org/10.1510/mmcts.2007.002758
Damiano, R. J., Jr., and R. K. Voeller. Biatrial lesion sets. J. Interv. Card. Electrophysiol. 20(3):95–99, 2007. https://doi.org/10.1007/s10840-007-9178-x
Gage, A. A., J. M. Baust, and J. G. Baust. Experimental cryosurgery investigations in vivo. Cryobiology. 59(3):229–243, 2009. https://doi.org/10.1016/j.cryobiol.2009.10.001
Hong, K. N., et al. Effect of epicardial fat on ablation performance: a three-energy source comparison. J. Card. Surg. 22(6):521–524, 2007. https://doi.org/10.1111/j.1540-8191.2007.00454.x
Khiabani, A. J., et al. The long-term outcomes and durability of the Cox-Maze IV procedure for atrial fibrillation. J. Thorac. Cardiovasc. Surg. 163:629, 2020. https://doi.org/10.1016/j.jtcvs.2020.04.100
Mack, M. J., et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 385(9986):2477–2484, 2015. https://doi.org/10.1016/s0140-6736(15)60308-7
McIntosh, R. L. A. A. A comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys. Rev. Lett. 5:129–151, 2010. https://doi.org/10.1142/S1793048010001184
Neel, H. B., III., and L. W. DeSanto. Cryosurgical control of cancer: effects of freeze rates, tumor temperatures, and ischemia. Ann. Otol. Rhinol. Laryngol. 82(5):716–723, 1973. https://doi.org/10.1177/000348947308200516
Neuber, J. U., F. Varghese, A. G. Pakhomov, and C. W. Zemlin. Using nanosecond shocks for cardiac defibrillation. Bioelectricity. 1(4):240–246, 2019. https://doi.org/10.1089/bioe.2019.0030
Neven, K., et al. Safety and feasibility of closed chest epicardial catheter ablation using electroporation. Circ. Arrhythm Electrophysiol. 7(5):913–919, 2014. https://doi.org/10.1161/CIRCEP.114.001607
Neven, K., et al. Acute and long-term effects of full-power electroporation ablation directly on the porcine esophagus. Circ. Arrhythm Electrophysiol. 2017. https://doi.org/10.1161/CIRCEP.116.004672
Nuccitelli, R., et al. Nanosecond pulsed electric fields cause melanomas to self-destruct. Biochem. Biophys. Res. Commun. 343(2):351–360, 2006. https://doi.org/10.1016/j.bbrc.2006.02.181
Nuccitelli, R., et al. A new pulsed electric field therapy for melanoma disrupts the tumor’s blood supply and causes complete remission without recurrence. Int. J. Cancer. 125(2):438–445, 2009. https://doi.org/10.1002/ijc.24345
Nuccitelli, R., K. Tran, S. Sheikh, B. Athos, M. Kreis, and P. Nuccitelli. Optimized nanosecond pulsed electric field therapy can cause murine malignant melanomas to self-destruct with a single treatment. Int. J. Cancer. 127(7):1727–1736, 2010. https://doi.org/10.1002/ijc.25364
Pakhomov, A. G., and O. N. Pakhomova. The interplay of excitation and electroporation in nanosecond pulse stimulation. Bioelectrochemistry.136:107598, 2020. https://doi.org/10.1016/j.bioelechem.2020.107598
Philpott, J. M., C. W. Zemlin, and R. J. Daminano. Surgical Treatment of Atrial Fibrillation. Cambridge: Academic Press, 2017
Pliquett, U., and R. Nuccitelli. Measurement and simulation of Joule heating during treatment of B-16 melanoma tumors in mice with nanosecond pulsed electric fields. Bioelectrochemistry. 100:62–68, 2014. https://doi.org/10.1016/j.bioelechem.2014.03.001
Prasad, S. M., et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J. Thorac. Cardiovasc. Surg. 126(6):1822–1828, 2003. https://doi.org/10.1016/s0022-5223(03)01287-x
Reddy, V. Y., et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin. Electrophysiol. 7(5):614–627, 2021. https://doi.org/10.1016/j.jacep.2021.02.014
Semenov, I., et al. Excitation and injury of adult ventricular cardiomyocytes by nano- to millisecond electric shocks. Sci. Rep. 8(1):8233, 2018. https://doi.org/10.1038/s41598-018-26521-2
Semenov, I., C. Zemlin, O. N. Pakhomova, S. Xiao, and A. G. Pakhomov. Diffuse, non-polar electropermeabilization and reduced propidium uptake distinguish the effect of nanosecond electric pulses. Biochim. Biophys. Acta. 1848(10 Pt A):2118–2125, 2015. https://doi.org/10.1016/j.bbamem.2015.06.018
van Es, R., M. H. A. Groen, M. Stehouwer, P. A. Doevendans, F. H. M. Wittkampf, and K. Neven. In vitro analysis of the origin and characteristics of gaseous microemboli during catheter electroporation ablation. J. Cardiovasc. Electrophysiol. 30(10):2071–2079, 2019. https://doi.org/10.1111/jce.14091
Varghese, F., J. U. Neuber, F. Xie, J. M. Philpott, A. G. Pakhomov, and C. W. Zemlin. Low-energy defibrillation with nanosecond electric shocks. Cardiovasc. Res. 113(14):1789–1797, 2017. https://doi.org/10.1093/cvr/cvx172
Voeller, R. K., A. Zierer, R. B. Schuessler, and R. J. Damiano Jr. Performance of a novel dual-electrode bipolar radiofrequency ablation device: a chronic porcine study. Innovations (Phila). 6(1):17–22, 2011. https://doi.org/10.1097/IMI.0b013e31820bc57f
Vyas, A., and Y. Lokhandwala. Coronary sinus as a site for stable temporary atrial pacing to tide over premature ventricular complex-triggered recurrent ventricular fibrillation in a patient with severe left ventricular dysfunction after coronary bypass surgery. Indian Heart J. 70(Suppl 3):S483–S485, 2018. https://doi.org/10.1016/j.ihj.2018.07.012
Weimar, T., et al. The cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ. Arrhythm Electrophysiol. 5(1):8–14, 2012. https://doi.org/10.1161/CIRCEP.111.963819
Weimar, T., A. M. Lee, S. Ray, R. B. Schuessler, and R. J. Damiano Jr. Evaluation of a novel cryoablation system: in vivo testing in a chronic porcine model. Innovations (Phila). 7(6):410–416, 2012. https://doi.org/10.1097/IMI.0b013e31828534e5
Xie, F., et al. Ablation of myocardial tissue with nanosecond pulsed electric fields. PLoS ONE.10(12):e0144833, 2015. https://doi.org/10.1371/journal.pone.0144833
Xie, F., and C. W. Zemlin. Effect of twisted fiber anisotropy in cardiac tissue on ablation with pulsed electric fields. PLoS ONE.11(4):e0152262, 2016. https://doi.org/10.1371/journal.pone.0152262
Zemlin, C. W. Safety factor for electrostimulation with nanosecond pulses. Bioelectrochemistry.141:107882, 2021. https://doi.org/10.1016/j.bioelechem.2021.107882
Conflict of interest
CZ holds stock in Pulse Biosciences.
Funding
This study was funded by a grant from Pulse Biosciences (Hayward, CA) to CZ.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Crystal Ripplinger oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Varghese, F., Philpott, J.M., Neuber, J.U. et al. Surgical Ablation of Cardiac Tissue with Nanosecond Pulsed Electric Fields in Swine. Cardiovasc Eng Tech 14, 52–59 (2023). https://doi.org/10.1007/s13239-022-00634-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-022-00634-2