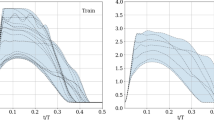
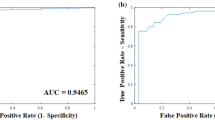
Abstract
Purpose
Patients receiving transcatheter aortic valve replacement (TAVR) can benefit from continuous, longitudinal monitoring of valve prosthesis to prevent leaflet thrombosis-related complications. We present a computational proof-of-concept study of a novel, non-invasive and non-toxic valve monitoring technique for TAVs which uses pressure measurements from microsensors embedded on the valve stent. We perform a data-driven analysis to determine the signal processing and machine learning required to detect reduced mobility in individual leaflets.
Methods
We use direct numerical simulations to describe hemodynamic differences in transvalvular flow in ascending aorta models with healthy and stenotic valves. A Cartesian-grid flow solver and a reduced-order valve model simulate the complex dynamics of blood flow and leaflet motion, respectively. The two-way fluid-structure interaction coupling is achieved using a sharp interface immersed boundary method.
Results
From a dataset of 21 simulations, we show leaflets with reduced mobility result in large, asymmetric pressure fluctuations in their vicinity, particularly in the region extending from the aortic sinus to the sino-tubular junction (STJ). We train a linear classifier algorithm by correlating sinus and STJ pressure measurements on the stent surface to individual leaflet status. The algorithm was shown to have >90% accuracy for prospective detection of individual leaflet dysfunction.
Conclusions
We demonstrate that using only two discrete pressure measurements, per leaflet, on the TAV stent, individual leaflet status can be accurately predicted. Such a sensorized TAV system could enable safe and inexpensive detection of prosthetic valve dysfunction.









Similar content being viewed by others
References
Allen, M. G. Micromachined endovascularly-implantable wireless aneurysm pressure sensors: from concept to clinic. In: The 13th International Conference on Solid-State Sensors, Actuators and Microsystems, 2005. Digest of Technical Papers. TRANSDUCERS ’05, 2005, volume 1, pp. 275–278.
Amat-Santos, I. J., D. Messika-Zeitoun, H. Eltchaninoff, S. Kapadia, S. Lerakis, A. N. Cheema et al. Infective endocarditis after transcatheter aortic valve implantation. Circulation 131:1566–1574, 2015.
Bailoor, S., J.-H. Seo, L. P. Dasi, S. Schena, and R. Mittal. A computational study of the hemodynamics of bioprosthetic aortic valves with reduced leaflet motion. J. Biomech. 120:110350, 2021.
Barletta, G., F. Venditti, P. Stefano, R. Del Bene, and C. Di Mario. Left ventricular outflow tract shape after aortic valve replacement with st jude trifecta prosthesis. Echocardiography 35:329–336, 2018.
Chakravarty, T., L. Søndergaard, J. Friedman, O. D. Backer, D. Berman, K. F. Kofoed et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. The Lancet 389:2383–2392, 2017.
Chen, L. Y., B. C.-K. Tee, A. L. Chortos, G. Schwartz, V. Tse, D. J. Lipomi et al. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat. Commun. 5:5028, 2014.
de Tullio, M. and G. Pascazio. A moving-least-squares immersed boundary method for simulating the fluid-structure interaction of elastic bodies with arbitrary thickness. J. Comput. Phys. 325:201–225, 2016.
Fonseca, M. A., M. G. Allen, J. Kroh, and J. White. Flexible wireless passive pressure sensors for biomedical applications. Solid-State Sensors, Actuators, and Microsystems Workshop, 2006, Hilton Head Island, South Carolina, 2006, pp. 37–42.
Généreux, P., S. J. Head, R. Hahn, B. Daneault, S. Kodali, M. R. Williams et al. Paravalvular leak after transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 61:1125–1136, 2013.
Gurvitch, R., A. Cheung, J. Ye, D. A. Wood, A. B. Willson, S. Toggweiler et al. Transcatheter valve-in-valve implantation for failed surgical bioprosthetic valves. J. Am. Coll. Cardiol. 58:2196–2209, 2011.
Gurvitch, R., D. Wood, E. Tay, J. Leipsic, J. Ye, S. Lichtenstein et al. Transcatheter aortic valve implantation. Circulation 122:1319–1327, 2010.
Hermans, M. C., M. S. Van Mourik, H. J. Hermens, J. Baan Jr, and M. M. Vis. Remote monitoring of patients undergoing transcatheter aortic valve replacement: a framework for postprocedural telemonitoring. JMIR Cardiol. 2:9, 2018.
Khalifa, A., Y. Liu, Y. Karimi, Q. Wang, A. Eisape, M. Stanaćević, N. Thakor, Z. Bao, and R. Etienne-Cummings. The microbead: A 0009 mm3 implantable wireless neural stimulator. IEEE Trans. Biomed. Circ. Syst. 13:971–985, 2019.
Kolte, D., G. J. Vlahakes, I. F. Palacios, R. Sakhuja, J. J. Passeri, I. Inglessis, and S. Elmariah. Transcatheter versus surgical aortic valve replacement in low-risk patients. J. Am. Coll. Cardiol. 74:1532–1540, 2019.
Kutz, J. N. Data-Driven Modeling & Scientific Computation: Methods for Complex Systems & Big Data, vol. 1 of 3, 1st edition. Oxford: Oxford University Press, Inc. (2013).
Makkar, R. R., G. Fontana, H. Jilaihawi, T. Chakravarty, K. F. Kofoed, O. De Backer et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N. Engl. J. Med. 373:2015–2024, 2015. PMID: 26436963.
Marcelli, E., B. Bortolani, I. Corazza, and L. Cercenelli. A novel sensorized heart valve prosthesis: preliminary in vitro evaluation. Sensors 18, 3905 (2018).
Marchena, E. D., J. Mesa, S. Pomenti, C. M. Y. Kall, X. Marincic, K. Yahagi et al. Thrombus formation following transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 8:728-739 (2015) TAVR Focus Issue.
Martí, D., M. Rubio, N. Escribano, R. De Miguel, I. Rada, and C. Morís. Very late thrombosis of a transcatheter aortic valve-in-valve. JACC Cardiovasc. Interv. 8:151–153, 2015.
Marwan, M., N. Mekkhala, M. Göller, J. Röther, D. Bittner, A. Schuhbaeck et al. Leaflet thrombosis following transcatheter aortic valve implantation. J. Cardiovasc. Comput. Tomogr. 12:8–13, 2018.
Mittal, R., H. Dong, M. Bozkurttas, F. Najjar, A. Vargas, and A. von Loebbecke. A versatile sharp interface immersed boundary method for incompressible flows with complex boundaries. J. Comput. Phys. 227:4825–4852, 2008.
Nishimura, R. A., C. M. Otto, R. O. Bonow, B. A. Carabello, J. P. Erwin, R. A. Guyton et al. 2014 aha/acc guideline for the management of patients with valvular heart disease: executive summary: a report of the american college of cardiology/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 63:2438–2488, 2014.
Pedley, T. Cardiovascular Fluid Mechanics. Vienna: Springer, pp. 1–72, 2003.
Reardon, M. J., N. M. Van Mieghem, J. J. Popma, N. S. Kleiman, L. Søndergaard, M. Mumtaz et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 376:1321–1331, 2017. PMID: 28304219.
Reul, H., A. Vahlbruch, M. Giersiepen, T. Schmitz-Rode, V. Hirtz, and S. Effert. The geometry of the aortic root in health, at valve disease and after valve replacement. J. Biomech. 23:181–191, 1990.
Rosseel, L., O. De Backer, and L. Søndergaard. Clinical valve thrombosis and subclinical leaflet thrombosis following transcatheter aortic valve replacement: Is there a need for a patient-tailored antithrombotic therapy? Front. Cardiovasc. Med. 6:44, 2019.
Seo, J. H., V. Vedula, T. Abraham, A. C. Lardo, F. Dawoud, H. Luo, and R. Mittal. Effect of the mitral valve on diastolic flow patterns. Phys. Fluids 26:121901, 2014.
Seo, J.-H., C. Zhu, J. Resar, and R. Mittal. Flow physics of normal and abnormal bioprosthetic aortic valves. Int. J. Heat Fluid Flow 86:108740, 2020.
Smith, C. R., M. B. Leon, M. J. Mack, D. C. Miller, J. W. Moses, L. G. Svensson et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 364:2187–2198, 2011. PMID: 21639811.
Vedula, V., J. Seo, A. Lardo, and R. Mittal. Effect of trabeculae and papillary muscles on the hemodynamics of the left ventricle. Theoret. Comput. Fluid Dyn. 30:3–21, 2016.
Xue, Q., R. Mittal, X. Zheng, and S. Bielamowicz. A computational study of the effect of vocal-fold asymmetry on phonation. J. Acous. Soc. Am. 128:818–827, 2010.
Zhou, Z. and R. Mittal. Swimming performance and unique wake topology of the sea hare (aplysia). Phys. Rev. Fluids 3:033102, 2018.
Zhu, C., J.-H. Seo, and R. Mittal. Computational modelling and analysis of haemodynamics in a simple model of aortic stenosis. J. Fluid Mech. 851:23–49, 2018.
Acknowledgements
This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation Grant Number TG-CTS100002. The authors acknowledge support from National Science Foundation award 1511200 and the Mirowski Discovery Award by the Johns Hopkins School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Human and Animal Studies
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Additional information
Associate Editor Igor Efimov oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bailoor, S., Seo, JH., Dasi, L. et al. Prosthetic Valve Monitoring via In Situ Pressure Sensors: In Silico Concept Evaluation using Supervised Learning. Cardiovasc Eng Tech 13, 90–103 (2022). https://doi.org/10.1007/s13239-021-00553-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-021-00553-8