Abstract
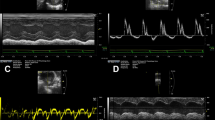
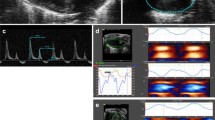
Decreased expression of elastin results in smaller, less compliant arteries, and high blood pressure. In mice, these differences become more significant with postnatal development. It is known that arterial size and compliance directly affect cardiac function, but the temporal changes in cardiac function have not been investigated in elastin insufficient mice. The aim of this study is to correlate changes in arterial size and compliance with cardiac function in wildtype (WT) and elastin haploinsufficient (Eln +/−) mice from birth to adulthood. Ultrasound scans were performed at the ages of 3, 7, 14, 21, 30, 60, and 90 days on male and female WT and Eln +/− mice. 2-D ultrasound and pulse wave Doppler images were used to measure the dimensions and function of the left ventricle (LV), ascending aorta, and carotid arteries. Eln +/− arteries are smaller and less compliant at most ages, with significant differences from WT as early as 3 days old. Surprisingly, there are no correlations (R 2 < 0.2) between arterial size and compliance with measures of LV hypertrophy or systolic function. There are weak correlations (0.2 < R 2 < 0.5) between arterial size and compliance with measures of LV diastolic function. Eln +/− mice have similar cardiac function to WT throughout postnatal development, demonstrating the remarkable ability of the developing cardiovascular system to adapt to mechanical and hemodynamic changes. Correlations between arterial size and compliance with diastolic function show that these measures may be useful indicators of early diastolic dysfunction.








Similar content being viewed by others
References
Adda, J., C. Mielot, R. Giorgi, F. Cransac, X. Zirphile, E. Donal, et al. Low-flow, low-gradient severe aortic stenosis despite normal ejection fraction is associated with severe left ventricular dysfunction as assessed by speckle-tracking echocardiography: a multicenter study. Circ. Cardiovasc. Imaging. 2011. doi:10.1161/CIRCIMAGING.111.967554.
Agoston-Coldea, L., T. Mocan, and C. Bobar. Arterial stiffness and left ventricular diastolic function in the patients with hypertension. Rom. J. Intern. Med. 46(4):313–321, 2008.
Baumann, P. Q., B. E. Sobel, A. K. Tarikuz Zaman, and D. J. Schneider. Gender-dependent differences in echocardiographic characteristics of murine hearts. Echocardiography 25(7):739–748, 2008. doi:10.1111/j.1540-8175.2008.00680.x.
Bose, A. K., J. W. Mathewson, B. E. Anderson, A. M. Andrews, A. Martin Gerdes, M. Benjamin Perryman, et al. Initial experience with high frequency ultrasound for the newborn C57BL mouse. Echocardiography 24(4):412–419, 2007.
Carasso, S., O. Cohen, D. Mutlak, Z. Adler, J. Lessick, D. Aronson, et al. Relation of myocardial mechanics in severe aortic stenosis to left ventricular ejection fraction and response to aortic valve replacement. Am. J. Cardiol. 107(7):1052–1057, 2011. doi:10.1016/j.amjcard.2010.11.032.
Chambers, J. The left ventricle in aortic stenosis: evidence for the use of ACE inhibitors. Heart 92(3):420–423, 2006. doi:10.1136/hrt.2005.074112.
Cheng, J. K., I. Stoilov, R. P. Mecham, and J. E. Wagenseil. A fiber-based constitutive model predicts changes in amount and organization of matrix proteins with development and disease in the mouse aorta. Biomech. Model. Mechanobiol. 2012. doi:10.1007/s10237-012-0420-9.
Ewart, A. K., C. A. Morris, G. J. Ensing, J. Loker, C. Moore, M. Leppert, et al. A human vascular disorder, supravalvular aortic stenosis, maps to chromosome 7. Proc. Natl Acad. Sci. U.S.A. 90(8):3226–3230, 1993.
Faury, G., M. Pezet, R. Knutsen, W. Boyle, S. Heximer, S. McLean, et al. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J. Clin. Invest. 112(9):1419–1428, 2003. doi:10.1172/JCI19028.
Finsen, A. V., G. Christensen, and I. Sjaastad. Echocardiographic parameters discriminating myocardial infarction with pulmonary congestion from myocardial infarction without congestion in the mouse. J. Appl. Physiol. 98(2):680–689, 2005. doi:10.1152/japplphysiol.00924.2004.
Fomovsky, G. M., S. Thomopoulos, and J. W. Holmes. Contribution of extracellular matrix to the mechanical properties of the heart. J. Mol. Cell. Cardiol. 48(3):490–496, 2010. doi:10.1016/j.yjmcc.2009.08.003.
Ghanem, A., W. Roll, T. Hashemi, O. Dewald, P. C. Djoufack, K. B. Fink, et al. Echocardiographic assessment of left ventricular mass in neonatal and adult mice: accuracy of different echocardiographic methods. Echocardiography 23(10):900–907, 2006. doi:10.1111/j.1540-8175.2006.00323.x.
Greenwald, S. E. Ageing of the conduit arteries. J. Pathol. 211(2):157–172, 2007. doi:10.1002/path.2101.
Greenwald, S. E., J. E. Moore, Jr., A. Rachev, T. P. Kane, and J. J. Meister. Experimental investigation of the distribution of residual strains in the artery wall. J. Biomech. Eng. 119(4):438–444, 1997.
Hamlin, S. K., P. S. Villars, J. T. Kanusky, and A. D. Shaw. Role of diastole in left ventricular function, II: diagnosis and treatment. Am. J. Crit. Care 13(6):453–466, 2004.
Hinton, Jr, R. B., C. M. Alfieri, S. A. Witt, B. J. Glascock, P. R. Khoury, D. W. Benson, et al. Mouse heart valve structure and function: echocardiographic and morphometric analyses from the fetus through the aged adult. Am. J. Physiol. Heart Circ. Physiol. 294(6):H2480–H2488, 2008. doi:10.1152/ajpheart.91431.2007.
Hirano, E., R. H. Knutsen, H. Sugitani, C. H. Ciliberto, and R. P. Mecham. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ. Res. 101(5):523–531, 2007.
Huang, Y., X. Guo, and G. S. Kassab. Axial nonuniformity of geometric and mechanical properties of mouse aorta is increased during postnatal growth. Am. J. Physiol. Heart Circ. Physiol. 290(2):H657–H664, 2006.
Hwang, J. W., S. J. Kang, H. S. Lim, B. J. Choi, S. Y. Choi, G. S. Hwang, et al. Impact of arterial stiffness on regional myocardial function assessed by speckle tracking echocardiography in patients with hypertension. J. Cardiovasc. Ultrasound 20(2):90–96, 2012. doi:10.4250/jcu.2012.20.2.90.
Jaroch, J., K. Loboz Grudzien, Z. Bociaga, A. Kowalska, E. Kruszynska, M. Wilczynska, et al. The relationship of carotid arterial stiffness to left ventricular diastolic dysfunction in untreated hypertension. Kardiologia polska. 70(3):223–231, 2012.
Johnson, G. L., J. M. Kotchen, H. E. McKean, C. M. Cottrill, and T. A. Kotchen. Blood pressure related echocardiographic changes in adolescents and young adults. Am. Heart J. 105(1):113–118, 1983.
Kelleher, C. M., S. E. McLean, and R. P. Mecham. Vascular extracellular matrix and aortic development. Curr. Top. Dev. Biol. 62:153–188, 2004.
Le, V., R. Knutsen, R. Mecham, and J. Wagenseil. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am. J. Physiol. Heart Circ. Physiol. 301(1):H221–H229, 2011. doi:10.1152/ajpheart.00119.2011.
Li, D. Y., B. Brooke, E. C. Davis, R. P. Mecham, L. K. Sorensen, B. B. Boak, et al. Elastin is an essential determinant of arterial morphogenesis. Nature 393(6682):276–280, 1998.
Li, D. Y., G. Faury, D. G. Taylor, E. C. Davis, W. A. Boyle, R. P. Mecham, et al. Novel arterial pathology in mice and humans hemizygous for elastin. J. Clin. Invest. 102(10):1783–1787, 1998.
Mahoney, L. T., R. M. Schieken, W. R. Clarke, and R. M. Lauer. Left ventricular mass and exercise responses predict future blood pressure. The Muscatine Study. Hypertension 12(2):206–213, 1988.
McEniery, C. M., I. B. Wilkinson, and A. P. Avolio. Age, hypertension and arterial function. Clin. Exp. Pharmacol. Physiol. 34(7):665–671, 2007. doi:10.1111/j.1440-1681.2007.04657.x.
Mujumdar, V. S., and S. C. Tyagi. Temporal regulation of extracellular matrix components in transition from compensatory hypertrophy to decompensatory heart failure. J. Hypertens. 17(2):261–270, 1999.
Nagueh, S. F. Echocardiographic assessment of left ventricular relaxation and cardiac filling pressures. Curr. Heart Failure Rep. 6(3):154–159, 2009.
Nagueh, S. F., C. P. Appleton, T. C. Gillebert, P. N. Marino, J. K. Oh, O. A. Smiseth, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 22(2):107–133, 2009. doi:10.1016/j.echo.2008.11.023.
Osborne, L. R., D. Martindale, S. W. Scherer, X. M. Shi, J. Huizenga, H. H. Heng, et al. Identification of genes from a 500-kb region at 7q11.23 that is commonly deleted in Williams syndrome patients. Genomics 36(2):328–336, 1996. doi:10.1006/geno.1996.0469.
Palmieri, V., J. N. Bella, M. J. Roman, E. Gerdts, V. Papademetriou, K. Wachtell, et al. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J. Hypertens. 21(4):781–787, 2003. doi:10.1097/01.hjh.0000052491.18130.dc.
Pettersen, M. D., W. Du, M. E. Skeens, and R. A. Humes. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J. Am. Soc. Echocardiogr. 21(8):922–934, 2008. doi:10.1016/j.echo.2008.02.006.
Pezet, M., M. P. Jacob, B. Escoubet, D. Gheduzzi, E. Tillet, P. Perret, et al. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res. 11(1):97–112, 2008.
Pollick, C., S. L. Hale, and R. A. Kloner. Echocardiographic and cardiac Doppler assessment of mice. J. Am. Soc. Echocardiogr. 8(5 Pt 1):602–610, 1995.
Scherrer-Crosbie, M., and H. B. Thibault. Echocardiography in translational research: of mice and men. J. Am. Soc. Echocardiogr. 21(10):1083–1092, 2008. doi:10.1016/j.echo.2008.07.001.
Schillaci, G., M. R. Mannarino, G. Pucci, M. Pirro, J. Helou, G. Savarese, et al. Age-specific relationship of aortic pulse wave velocity with left ventricular geometry and function in hypertension. Hypertension 49(2):317–321, 2007. doi:10.1161/01.HYP.0000255790.98391.9b.
Shapiro, L. M., and D. G. Gibson. Patterns of diastolic dysfunction in left ventricular hypertrophy. Br. Heart J. 59(4):438–445, 1988.
Urbina, E. M., S. S. Gidding, W. Bao, A. S. Pickoff, K. Berdusis, and G. S. Berenson. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation 91(9):2400–2406, 1995.
Vinereanu, D., E. Nicolaides, L. Boden, N. Payne, C. J. Jones, and A. G. Fraser. Conduit arterial stiffness is associated with impaired left ventricular subendocardial function. Heart 89(4):449–450, 2003.
Vriz, O., E. Bossone, M. Bettio, D. Pavan, S. Carerj, and F. Antonini-Canterin. Carotid artery stiffness and diastolic function in subjects without known cardiovascular disease. J. Am. Soc. Echocardiogr. 24(8):915–921, 2011. doi:10.1016/j.echo.2011.05.001.
Wagenseil, J. E., C. H. Ciliberto, R. H. Knutsen, M. A. Levy, A. Kovacs, and R. P. Mecham. Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ. Res. 104(10):1217–1224, 2009. doi:10.1161/CIRCRESAHA.108.192054.
Wagenseil, J. E., N. L. Nerurkar, R. H. Knutsen, R. J. Okamoto, D. Y. Li, and R. P. Mecham. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am. J. Physiol. Heart Circ. Physiol. 289(3):H1209–H1217, 2005. doi:10.1152/ajpheart.00046.2005.
Wan, W., H. Yanagisawa, and R. L. Gleason, Jr. Biomechanical and microstructural properties of common carotid arteries from fibulin-5 null mice. Ann. Biomed. Eng. 38(12):3605–3617, 2010. doi:10.1007/s10439-010-0114-3.
Webster, A., Le, V. P., and Wagenseil, J. E., editors. Quantifying elastin, collagen and total protein in mouse arteries. In: Biomedical Engineering Society Annual Meeting, Austin, TX, 2010.
Yanagisawa, H., E. C. Davis, B. C. Starcher, T. Ouchi, M. Yanagisawa, J. A. Richardson, et al. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415(6868):168–171, 2002.
Acknowledgments
This work was funded, in part, by National Institutes of Health grants R00 HL087653, R01 HL105314, and R01 HL115560. Dr. Robert Mecham at the Washington University School of Medicine is gratefully acknowledged for providing the Eln +/− mice. The Saint Louis University Center for Cardiovascular Research is gratefully acknowledged for providing the ultrasound equipment.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Le, V.P., Wagenseil, J.E. Echocardiographic Characterization of Postnatal Development in Mice with Reduced Arterial Elasticity. Cardiovasc Eng Tech 3, 424–438 (2012). https://doi.org/10.1007/s13239-012-0108-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-012-0108-4