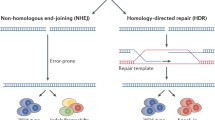
Abstract
Gene editing and therapy holds immense promise in addressing cardiovascular disorders by enabling precise modifications to the genetic groundworks of these conditions. This technology offers a targeted approach to correct or mitigate not only the genetic mutations that are responsible for inherited cardiovascular diseases, such as hypertrophic cardiomyopathy or familial hypercholesterolemia (FH), but also in controlling and regulating the genes in conditions that are age-related or that arise due to unnecessary gene activity, which often lack effective treatments. By editing the relevant genes, we aim to reduce disease severity, improve heart function, prevent future episodes and potentially improve the health span. Recent trials involving gene therapy and gene editing in cardiovascular disorders have revolutionized treatment strategies, offering hope for patients with genetic predispositions to heart and vessel-related ailments and advancing the pursuit for more personalized and effective therapies. In this review, we have consolidated the genetic mutations causing cardiovascular diseases (CVDs) followed by latest advancements in the gene editing technologies and their therapeutic implications along with involved ethical challenges and risk factors.
Graphical Abstract

Similar content being viewed by others
Abbreviations
- AAV:
-
Adeno associated virus
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- Cas:
-
CRISPR associated protein
- CVDs:
-
Cardiovascular diseases
- iPSC:
-
Induced pluripotent stem cells
- FH:
-
Familial hypercholesterolemia
- PAH:
-
Pulmonary arterial hypertension
- FTAAD:
-
Familial thoracic aortic aneurysm and dissection
- ZFNs:
-
Zinc-finger nucleases
- PAM:
-
Protospacer adjacent motif
- HCM:
-
Hypertrophic cardiomyopathy
- sgRNA:
-
Single-guide RNA
- crRNA:
-
CRISPR-RNAs
- tracrRNA:
-
trans-activating CrRNA
- TALENS:
-
Transcription activator–like effector nucleases
- VSMCs:
-
Vascular smooth muscle cells
References
Anastasiou M, Oikonomou E, Theofilis P, et al. Prolonged impact of anti-cancer therapy on endothelial function and arterial stiffness in breast cancer patients. Vasc Pharmacol. 2023;152:107195. https://doi.org/10.1016/j.vph.2023.107195.
Andreassi MG. Coronary atherosclerosis and somatic mutations: an overview of the contributive factors for oxidative DNA damage. Mutat Res Rev Mutat Res. 2003;543:67–86.
Ballinger SW, Patterson C, Yan C, et al. Mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960–7.
Basso C, Corrado D, Rossi L, Thiene G. Ventricular preexcitation in children and young adults: atrial myocarditis as a possible trigger of sudden death. Circulation. 2001;103:269–75. https://doi.org/10.1161/01.CIR.103.2.269.
Bennett MR, Evan GI, Schwartz SM. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995;95:2266–74. https://doi.org/10.1172/JCI117917.
Botto N, Masetti S, Petrozzi L, et al. Elevated levels of oxidative DNA damage in patients with coronary artery disease. Coron Artery Dis. 2002;13:269–74. https://doi.org/10.1097/00019501-200208000-00004.
Brouns SJJ, Jore MM, Lundgren M, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–4. https://doi.org/10.1126/SCIENCE.1159689.
Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. https://doi.org/10.1161/CIRCULATIONAHA.113.001878.
Cannatà A, Ali H, Sinagra G, Giacca M. Gene therapy for the heart lessons learned and future perspectives. Circ Res. 2020. https://doi.org/10.1161/CIRCRESAHA.120.315855.
Cao G, Xuan X, Zhang R, et al. Gene Therapy for Cardiovascular Disease: Basic Research and Clinical Prospects. Front Cardiovasc Med. 2021;8:760140. https://doi.org/10.3389/FCVM.2021.760140.
Cardus A, Uryga AK, Walters G, Erusalimsky JD. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc Res. 2013;97:571–9. https://doi.org/10.1093/cvr/cvs352.
Cui M, Wang Z, Bassel-Duby R, Olson EN. Genetic and epigenetic regulation of cardiomyocytes in development, regeneration and disease. Development. 2018. https://doi.org/10.1242/DEV.171983.
Devetzi M, Goulielmaki M, Khoury N, et al. Genetically-modified stem cells in treatment of human diseases: tissue kallikrein (KLK1)-based targeted therapy (Review). Int J Mol Med. 2018;41:1177–86. https://doi.org/10.3892/IJMM.2018.3361.
Ding Q, Strong A, Patel KM, et al. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ Res. 2014;115:488–92. https://doi.org/10.1161/CIRCRESAHA.115.304351/-/DC1.
Doetschman T, Azhar M. Cardiac-specific inducible and conditional gene targeting in mice. Circ Res. 2012;110:1498–512. https://doi.org/10.1161/CIRCRESAHA.112.265066.
Dorsheimer L, Assmus B, Rasper T, et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 2019;4:32. https://doi.org/10.1001/JAMACARDIO.2018.3965.
Evans MA, Sano S, Walsh K. Cardiovascular disease, aging, and clonal hematopoiesis. Annu Rev Pathol. 2019. https://doi.org/10.1146/annurev-pathmechdis.
Flouris GA, Arvanitis DA, Parissis JT, et al. Loss of heterozygosity in DNA mismatch repair genes in human atherosclerotic plaques. Mol Cell Biol Res Commun. 2001;4:62–5. https://doi.org/10.1006/mcbr.2000.0255.
Foglia MJ, Poss KD. Building and re-building the heart by cardiomyocyte proliferation. Development. 2016;143(5):729–40. https://doi.org/10.1242/dev.132910.
Fu Y, Foden JA, Khayter C, et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31:822–6. https://doi.org/10.1038/nbt.2623.
Gasiunas G, Barrangou R, Horvath P, Siksnys V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci U S A. 2012. https://doi.org/10.1073/PNAS.1208507109/-/DCSUPPLEMENTAL/PNAS.201208507SI.PDF.
Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–87. https://doi.org/10.1056/NEJMOA1409405.
German DM, Mitalipov S, Mishra A, Kaul S. Therapeutic genome editing in cardiovascular diseases. JACC Basic Transl Sci. 2019;4:122–31. https://doi.org/10.1016/j.jacbts.2018.11.004.
Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118:6072–8. https://doi.org/10.1002/cncr.27633.Percutaneous.
Gollob MH, Green MS, Tang AS-L, et al. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001;344:1823–31. https://doi.org/10.1056/NEJM200106143442403.
Gollob MH, Green MS, Tang ASL, Roberts R. PRKAG2 cardiac syndrome: familial ventricular preexcitation, conduction system disease, and cardiac hypertrophy. Curr Opin Cardiol. 2002;17:229–34. https://doi.org/10.1097/00001573-200205000-00004.
Gong J, Zhou D, Jiang L, et al. In vitro lineage-specific differentiation of vascular smooth muscle cells in response to SMAD3 deficiency: implications for SMAD3-related thoracic aortic aneurysm. Arterioscler Thromb Vasc Biol. 2020;40:1651–63. https://doi.org/10.1161/ATVBAHA.120.313033.
Gray K, Kumar S, Figg N, et al. Effects of DNA damage in smooth muscle cells in atherosclerosis. Circ Res. 2014;116(5):816–26. https://doi.org/10.1161/CIRCRESAHA.116.304921.
Grossman M, Rader DJ, Muller DWM, et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995;111(1):1148–54. https://doi.org/10.1038/nm1195-1148.
Gwathmey JK, Yerevanian A, Hajjar RJ. Cardiac gene therapy with SERCA2a: from bench to bedside. J Mol Cell Cardiol. 2010;50(5):803–12. https://doi.org/10.1016/j.yjmcc.2010.11.011.
Halcox JPJ. Endothelial dysfunction. Prim Auton Nerv Syst. 2012;12:319–24. https://doi.org/10.1016/B978-0-12-386525-0.00066-4.
Hall AE, Burton H. Legal and ethical implications of inherited cardiac disease in clinical practice within the UK. J Med Ethics. 2010;36:762–6. https://doi.org/10.1136/jme.2009.034108.
Hatzistamou J, Kiaris H, Ergazaki M, Spandidos DA. Loss of heterozygosity and microsatellite instability in human atherosclerotic plaques. Biochem Biophys Res Commun. 1996;225:186–90. https://doi.org/10.1006/bbrc.1996.1151.
Hennig SL, Owen JR, Lin JC, et al. Evaluation of mutation rates, mosaicism and off target mutations when injecting Cas9 mRNA or protein for genome editing of bovine embryos. Sci Rep. 2020;10(1):22309. https://doi.org/10.1038/s41598-020-78264-8.
Higo T, Naito AT, Sumida T, et al. DNA single-strand break-induced DNA damage response causes heart failure. Nat Commun. 2017;8:1–13. https://doi.org/10.1038/ncomms15104.
How to respond to CRISPR babies. Nature. 2018;564:5. https://doi.org/10.1038/d41586-018-07634-0
Huang Y, Hong H, Li M, et al. Age-dependent oxidative DNA damage does not correlate with reduced proliferation of cardiomyocytes in humans. PLoS ONE. 2017;12(1):1–13. https://doi.org/10.1371/journal.pone.0170351.
Ishikawa K, Weber T, Hajjar RJ. Human cardiac gene therapy. Circ Res. 2018;123:601–13. https://doi.org/10.1161/CIRCRESAHA.118.311587.
Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;26:2488–98. https://doi.org/10.1056/NEJMoa1408617.
Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–21. https://doi.org/10.1056/NEJMOA1701719.
Jean D, Peter G. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32. https://doi.org/10.1161/01.CIR.0000131515.03336.f8.
Jiang F, Doudna JA. CRISPR–Cas9 structures and mechanisms. Annu Rev Biophys. 2017;46:505–29. https://doi.org/10.1146/ANNUREV-BIOPHYS-062215-010822.
Jinek M, Chylinski K, Fonfara I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21. https://doi.org/10.1126/SCIENCE.1225829.
Khan M, Völkers M, Wende AR. Editorial: metabolic regulation of cardiac and vascular cell function: physiological and pathophysiological implications. Front Physiol. 2022;13: 849869. https://doi.org/10.3389/FPHYS.2022.849869.
Khouzam JPS, Tivakaran VS. CRISPR-Cas9 applications in cardiovascular disease. Curr Probl Cardiol. 2021;46: 100652. https://doi.org/10.1016/J.CPCARDIOL.2020.100652.
Kim SW, Lee DW, Yu LH, et al. Mesenchymal stem cells overexpressing GCP-2 improve heart function through enhanced angiogenic properties in a myocardial infarction model. Cardiovasc Res. 2012;95:495–506. https://doi.org/10.1093/CVR/CVS224.
Knott GJ, Doudna JA. CRISPR-Cas guides the future of genetic engineering. Science. 2018;361:866–9. https://doi.org/10.1126/SCIENCE.AAT5011.
Kurose I, Wolf R, Cerwinka W, Granger DN. Microvascular responses to ischemia/reperfusion in normotensive and hypertensive rats. Hypertension. 1999;34:212–6. https://doi.org/10.1161/01.HYP.34.2.212.
Landis BJ, Veldtman GR, Ware SM. Genotype-phenotype correlations in Marfan syndrome. Heart. 2017;103:1750–2. https://doi.org/10.1136/heartjnl-2017-311513.
Lewandoski M. Conditional control of gene expression in the mouse. Nature Rev Genet. 2002;2(10):743–55.
Li ZH, Wang J, Xu JP, et al. (2023) Recent advances in CRISPR-based genome editing technology and its applications in cardiovascular research. Mil Med Res. 2023;101(10):1–20. https://doi.org/10.1186/S40779-023-00447-X.
Li Q, Zhang A, Tao C, et al. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2013;441:675–80. https://doi.org/10.1016/J.BBRC.2013.10.071.
Liu N, Olson EN. CRISPR modeling and correction of cardiovascular disease. Circ Res. 2022;130:1827–50. https://doi.org/10.1161/CIRCRESAHA.122.320496.
Lloyd-Jones DM, Nam BH, D’Agostino RB, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291:2204–11. https://doi.org/10.1001/JAMA.291.18.2204.
Long C, Li H, Tiburcy M, et al. Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci Adv. 2018. https://doi.org/10.1126/SCIADV.AAP9004/SUPPL_FILE/AAP9004_SM.PDF.
Long L, Ormiston ML, Yang X, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–85. https://doi.org/10.1038/nm.3877.
Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–51. https://doi.org/10.1056/NEJM198610233151702.
Luo R, Lu Y, Liu J, et al. Enhancement of the efficacy of mesenchymal stem cells in the treatment of ischemic diseases. Biomed Pharmacother. 2019;109:2022–34. https://doi.org/10.1016/J.BIOPHA.2018.11.068.
Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, Koski A, Ji D, Hayama T, Ahmed R, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–9. https://doi.org/10.1038/nature23305.
Machado RD, Aldred MA, James V, et al. Mutations of the TGF-β type II receptorBMPR2 in pulmonary arterial hypertension. Hum Mutat. 2006;27:121–32. https://doi.org/10.1002/humu.20285.
Mao Z, Hine C, Tian X, et al. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–6. https://doi.org/10.1126/science.1202723.
Martinet W, Knaapen MWM, De Meyer GRY, et al. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–32. https://doi.org/10.1161/01.CIR.0000026393.47805.21.
Matthews C, Gorenne I, Scott S, et al. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–64. https://doi.org/10.1161/01.RES.0000233315.38086.bc.
Mendell JR, Al-Zaidy SA, Rodino-Klapac LR, et al. Current clinical applications of in vivo gene therapy with AAVs. Mol Ther. 2021;29:464–88. https://doi.org/10.1016/J.YMTHE.2020.12.007.
Mensah GA, Moran AE, Roth GA, Narula J. The global burden of cardiovascular diseases, 1990–2010. Glob Heart. 2014;9:183–4. https://doi.org/10.1016/j.gheart.2014.01.008.
Mercer J, Mahmoudi M, Bennett M. DNA damage, p53, apoptosis and vascular disease. Mutat Res Fundam Mol Mech Mutagen. 2007;621:75–86. https://doi.org/10.1016/j.mrfmmm.2007.02.011.
Moore OM, Ho KS, Copeland JS, et al. Genome editing and cardiac arrhythmias. Cells. 2023. https://doi.org/10.3390/cells12101363.
Motta BM, Pramstaller PP, Hicks AA, Rossini A. The impact of CRISPR/Cas9 technology on cardiac research: from disease modelling to therapeutic approaches. Stem Cells Int. 2017. https://doi.org/10.1155/2017/8960236.
Musunuru K, Chadwick AC, Mizoguchi T, et al. (2021) In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature. 2021;5937859(593):429–34. https://doi.org/10.1038/s41586-021-03534-y.
Naim C, Yerevanian A, Hajjar RJ. Gene therapy for heart failure: Where do we stand? Curr Cardiol Rep. 2013;15:1–10. https://doi.org/10.1007/s11886-012-0333-3.
Nakada Y, Nhi Nguyen NU, Xiao F, et al. DNA damage response mediates pressure overload-induced cardiomyocyte hypertrophy. Circulation. 2019;139:1237–9. https://doi.org/10.1161/CIRCULATIONAHA.118.034822.
Nakamura Y, Kita S, Tanaka Y, et al. Adiponectin stimulates exosome release to enhance mesenchymal stem-cell-driven therapy of heart failure in mice. Mol Ther. 2020;28:2203–19. https://doi.org/10.1016/j.ymthe.2020.06.026.
National Academies of Sciences, Engineering, and Medicine. Human genome editing: science, ethics, and governance. Washington: The National Academies Press; 2017. https://doi.org/10.17226/24623.
Octavia Y, Tocchetti CG, Gabrielson KL, et al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52(6):1213–25. https://doi.org/10.1016/j.yjmcc.2012.03.006.
Olson EN. Toward the correction of muscular dystrophy by gene editing. Proc Natl Acad Sci USA. 2021. https://doi.org/10.1073/PNAS.2004840117.
Van Der Oost J, Westra ER, Jackson RN, Wiedenheft B. Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nat Rev Microbiol. 2014;12:479–92. https://doi.org/10.1038/NRMICRO3279.
Ormond KE, Borensztein MJ, Hallquist MLG, et al. Defining the critical components of informed consent for genetic testing. J Pers Med. 2021. https://doi.org/10.3390/jpm11121304.
Pan X, Philippen L, Lahiri SK, et al. In Vivo Ryr2 editing corrects catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2018;123:953–63. https://doi.org/10.1161/CIRCRESAHA.118.313369.
Paquette M, Baass A. A novel cause of familial hypercholesterolemia: PCSK9 gene duplication. Can J Cardiol. 2018;34:1259–60. https://doi.org/10.1016/j.cjca.2018.08.027.
Puente BN, Kimura W, Muralidhar SA, et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–79. https://doi.org/10.1016/j.cell.2014.03.032.
Reiner AP, Bhattacharya R, Zekavat SM, et al. Clonal hematopoiesis is associated with higher risk of stroke. Stroke. 2022;53:788–97. https://doi.org/10.1161/STROKEAHA.121.037388.
Renard M, Francis C, Ghosh R, et al. Clinical validity of genes for heritable thoracic aortic aneurysm and dissection. J Am Coll Cardiol. 2018;72:605–15. https://doi.org/10.1016/j.jacc.2018.04.089.
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. https://doi.org/10.1038/362801a0.
Rouet P, Smih F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994;14:8096–106.
Satoh M, Ishikawa Y, Takahashi Y, et al. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198:347–53. https://doi.org/10.1016/j.atherosclerosis.2007.09.040.
Schreurs J, Sacchetto C, Colpaert RMW, et al. Recent advances in crispr/cas9-based genome editing tools for cardiac diseases. Int J Mol Sci. 2021;22:10985. https://doi.org/10.3390/ijms222010985.
Shah A, Gray K, Figg N, et al. Defective base excision repair of oxidative DNA damage in vascular smooth muscle cells promotes atherosclerosis. Circulation. 2018;138:1446–62. https://doi.org/10.1161/CIRCULATIONAHA.117.033249.
Shukla PC, Singh KK, Yanagawa B, et al. DNA damage repair and cardiovascular diseases. Can J Cardiol. 2010;26:13A-16A. https://doi.org/10.1016/S0828-282X(10)71055-2.
Tian W, Jiang X, Sung YK, et al. Phenotypically silent bone morphogenetic protein receptor 2 mutations predispose rats to inflammation-induced pulmonary arterial hypertension by enhancing the risk for neointimal transformation. Circulation. 2019;140:1409–25. https://doi.org/10.1161/CIRCULATIONAHA.119.040629.
Torres-Ruiz R, Rodriguez-Perales S. CRISPR-Cas9 technology: applications and human disease modelling. Brief Funct Genomics. 2017;16:4–12. https://doi.org/10.1093/BFGP/ELW025.
Touyz RM. Oxidative stress and vascular damage in hypertension. Curr Hypertens Rep. 2000;2(1):98–105.
Verhaart IEC, Aartsma-Rus A. Therapeutic developments for Duchenne muscular dystrophy. Nat Rev Neurol. 2019;15(7):373–86.
Wang D, Zhang F, Gao G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181:136–50. https://doi.org/10.1016/J.CELL.2020.03.023.
Wenzel P, Schuhmacher S, Kienhöfer J, et al. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res. 2008;80:280–9. https://doi.org/10.1093/cvr/cvn182.
Wolf CM. Hypertrophic cardiomyopathy: genetics and clinical perspectives. Cardiovasc Diagn Ther. 2019;9:S388. https://doi.org/10.21037/CDT.2019.02.01.
Xie C, Zhang YP, Song L, Luo J, Qi W, Hu J, Lu D, Yang Z, Zhang J, Xiao J, Zhou B, Du JL. Genome editing with CRISPR/Cas9 in postnatal mice corrects PRKAG2 cardiac syndrome. Cell Res. 2016;26(10):1099–111.
Xu S, Yin M, Koroleva M, et al. SIRT6 protects against endothelial dysfunction and atherosclerosis in mice. Aging (Albany NY). 2016;8:1064–82. https://doi.org/10.18632/aging.100975.
Zeng Y, Li J, Li G, et al. Correction of the marfan syndrome pathogenic FBN1 mutation by base editing in human cells and heterozygous embryos. Mol Ther. 2018;26:2631–7. https://doi.org/10.1016/J.YMTHE.2018.08.007.
Zhang Y, Li H, Min YL, et al. Enhanced CRISPR-Cas9 correction of Duchenne muscular dystrophy in mice by a self-complementary AAV delivery system. Sci Adv. 2020;6:6812. https://doi.org/10.1126/SCIADV.AAY6812/SUPPL_FILE/AAY6812_SM.PDF.
Zhao H, Li Y, He L, et al. In vivo AAV-CRISPR/Cas9–mediated gene editing ameliorates atherosclerosis in familial hypercholesterolemia. Circulation. 2020;141:67–79. https://doi.org/10.1161/CIRCULATIONAHA.119.042476.
Funding
This work did not receive any funding.
Author information
Authors and Affiliations
Contributions
DS, RC, PS, and DG—wrote the manuscript. PCS—ideation and finalization of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Financial interests: RC and SD received no financial support for this work. Non-financial interests: The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Editor: Nishant Chakravorty; Reviewers: Syamantak Majumder, Amit Kumar Pandey.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duddu, S., Chakrabarti, R., Sharma, P. et al. Gene editing and therapy in acquired and inherited cardiovascular disorders. Nucleus 67, 237–250 (2024). https://doi.org/10.1007/s13237-024-00480-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-024-00480-8