Abstract
One of the most prevalent neurological movement diseases affecting the geriatric population globally is Parkinson’s disease (PD). Recent studies have highlighted the potency of biomolecules in the generation of nanomaterials and also over their impact on neuroprotection. The objective of this research was to investigate the potential of iron oxide nanoparticles produced using ascorbic acid (AA-IONPs) against PD. Numerous analytical methods including UV–Vis analysis, Fourier-Transform Infrared Spectroscopy (FTIR), dynamic light scattering (DLS), and electron microscopy (SEM, TEM), were used to analyze the produced AA-IONPs. Nitric oxide, prostaglandin E2, and inflammatory cytokines analyses such as IL-6 and IL-1 were employed to assess the neuroprotective effect of synthesized AA-IONPs on inflammatory agent lipopolysaccharides driven murine microglial BV2 cells. And also Parkinson-induced C57BL/6 mice were given the nanoparticle treatment to confirm the in vivo effects of the produced nanoparticles. Our characterization findings had demonstrated that AA-IONPs have a significant role in acting as an ideal nano drug and may have the ability to reduce inflammation in in vitro murine microglial BV2. The outcomes of in vivo tests conclusively show that AA-IONPs had reduced neuroinflammation and enhanced motor coordination in Parkinson’s disease-induced rats.
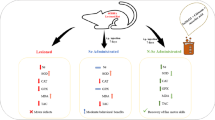
Graphical abstract

Ascorbic acid mediated synthesis of iron nanoparticles and its neuroprotective activity against MPTP induced mice model. Iron oxide nanoparticles synthesized using through ascorbic as reducer cum stabilizing agent, then it is characterized with UV-Vis spec, FTIR, XRD, SEM and TEM. The synthesized AA-IONPs are highly crystalline as well biologically more effective. MPTP was used to induce the neural inflammation and then the AA-IONPS treatment carried out. The Experiment outcome shows the AA-IONPs very active on damaged neuron further tend to recovery the axonal potential and neuroplasmic integration on treated neural cells. Over all the study augment that AA-IONPs could efficiently protect the neural damage via preventing pro-inflammatory signal inhibition and to could use for further neuroprotective drug development














Similar content being viewed by others
References
A. Oueslati, Implication of alpha-synuclein phosphorylation at S129 in synucleinopathies: what have we learned in the last decade? J. Parkinsons. Dis. 6, 39–51 (2016)
G. Vidal-Martinez, K. Najera, J.D. Miranda et al., FTY720 improves behavior, increases brain derived neurotrophic factor levels and reduces α-synuclein pathology in Parkinsonian GM2 +/− Mice. Neuroscience 411, 1–10 (2019). https://doi.org/10.1016/j.neuroscience.2019.05.029
J. Xue, T. Liu, Y. Liu et al., Neuroprotective effect of biosynthesised gold nanoparticles synthesised from root extract of Paeonia moutan against Parkinson disease—in vitro & in vivo model. J Photochem Photobiol B Biol. 200, 111635 (2019). https://doi.org/10.1016/j.jphotobiol.2019.111635
M. Lotia, J. Jankovic, New and emerging medical therapies in Parkinsons disease. Expert Opin. Pharmacother. 17, 895–909 (2016)
B.D. Li, Z.Y. Bi, J.F. Liu et al., Adverse effects produced by different drugs used in the treatment of Parkinson’s disease: a mixed treatment comparison. CNS Neurosci Ther 23, 827–842 (2017). https://doi.org/10.1111/cns.12727
M.M. Yallapu, S.F. Othman, E.T. Curtis et al., Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 32, 1890–1905 (2011). https://doi.org/10.1016/j.biomaterials.2010.11.028
P. Tartaj, M.M. Del Puerto, S. Veintemillas-Verdaguer et al., The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D. Appl. Phys. 36, R182 (2003)
F. Böttger, A. Vallés-Martí, L. Cahn, C.R. Jimenez, High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J. Exp. Clin. Cancer Res. 40, 1–44 (2021)
B. Ngo, J.M. Van Riper, L.C. Cantley, J. Yun, Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer 19, 271–282 (2019). https://doi.org/10.1038/s41568-019-0135-7
S.C. Fletcher, M.L. Coleman, Human 2-oxoglutarate-dependent oxygenases: nutrient sensors, stress responders, and disease mediators. Biochem. Soc. Trans. 48, 1843–1858 (2020)
T. Lee Chong, E.L. Ahearn, L. Cimmino, Reprogramming the epigenome with vitamin C. Front. Cell Dev. Biol. 7, 128 (2019)
A. Ang, J.M. Pullar, M.J. Currie, M.C.M. Vissers, Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 46, 1147–1159 (2018)
K. Kavithaa, M. Paulpandi, P.R. Padma, S. Sumathi, Induction of intrinsic apoptotic pathway and cell cycle arrest: Via baicalein loaded iron oxide nanoparticles as a competent nano-mediated system for triple negative breast cancer therapy. RSC Adv 6, 64531–64543 (2016). https://doi.org/10.1039/c6ra11658b
T. Ponraj, M. Paulpandi, R. Vivek et al., Protein regulation and apoptotic induction in human breast carcinoma cells (MCF-7) through lectin from G. beauts. Int J Biol Macromol 95, 1235–1245 (2017). https://doi.org/10.1016/j.ijbiomac.2016.11.018
M. Haghighat, H.Q. Alijani, M. Ghasemi et al., Cytotoxicity properties of plant-mediated synthesized K-doped ZnO nanostructures. Bioprocess Biosyst Eng 45, 97–105 (2022). https://doi.org/10.1007/s00449-021-02643-2
V.A. Niraimathee, V. Subha, R.S. Ernest Ravindran, S. Renganathan, Green synthesis of iron oxide nanoparticles from Mimosa pudica root extract. Int J Environ Sustain Dev 15, 227–240 (2016). https://doi.org/10.1504/IJESD.2016.077370
A. Umer, S. Naveed, N. Ramzan et al., A green method for the synthesis of copper nanoparticles using l-ascorbic acid. Rev Mater 19, 197–203 (2014). https://doi.org/10.1590/S1517-70762014000300002
P. Balu, I.V. Asharani, D. Thirumalai, Catalytic degradation of hazardous textile dyes by iron oxide nanoparticles prepared from Raphanus sativus leaves’ extract: a greener approach. J Mater Sci Mater Electron 31, 10669–10676 (2020). https://doi.org/10.1007/s10854-020-03616-z
J. Sandhya, S. Kalaiselvam, Biogenic synthesis of magnetic iron oxide nanoparticles using inedible borassus flabellifer seed coat: characterization, antimicrobial, antioxidant activity and in vitro cytotoxicity analysis. Mater Res Express (2020). https://doi.org/10.1088/2053-1591/ab6642
A. Sood, V. Arora, J. Shah et al., Ascorbic acid-mediated synthesis and characterisation of iron oxide/gold core–shell nanoparticles. J Exp Nanosci 11, 370–382 (2016). https://doi.org/10.1080/17458080.2015.1066514
D. Leybo, M. Tagirov, E. Permyakova et al., Ascorbic acid-assisted polyol synthesis of iron and Fe/Go, Fe/h-BN composites for Pb2+ removal from wastewaters. Nanomaterials 10, 37 (2020). https://doi.org/10.3390/nano10010037
R.M. Elamawi, R.E. Al-Harbi, A.A. Hendi, Biosynthesis and characterization of silver nanoparticles using trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt J Biol Pest Control 28, 1–11 (2018). https://doi.org/10.1186/s41938-018-0028-1
Z. Izadiyan, K. Shameli, M. Miyake et al., Cytotoxicity assay of plant-mediated synthesized iron oxide nanoparticles using Juglans regia green husk extract. Arab J Chem 13, 2011–2023 (2020). https://doi.org/10.1016/j.arabjc.2018.02.019
Y. Asefi, R. Fahimi, S. Ghorbian, Synergistic effect of vitamin c with superparamagnetic iron oxide nanoparticles for inhibiting proliferation of gastric cancer cells. Biointerface Res Appl Chem. 12, 3215–3224 (2022). https://doi.org/10.33263/BRIAC123.32153224
G. Abbas, K.B. Singh, N. Kumar et al., Efficient anticarcinogenic activity of α-Fe2O3 nanoparticles: In-vitro and computational study on human renal carcinoma cells HEK-293. Mater Today Commun. 26, 102175 (2021). https://doi.org/10.1016/j.mtcomm.2021.102175
P. Janhom, P. Dharmasaroja, Neuroprotective effects of alpha-mangostin on MPP+-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. J Toxicol. (2015). https://doi.org/10.1155/2015/919058
Q. Li, Y. Wu, X.S. Chen et al., Ascorbic acid 6-palmitate modulates microglia M1/M2 polarization in lipopolysaccharide-stimulated BV-2 cells via PERK/elF2α mediated endoplasmic reticulum stress. BMC Complement Med Ther 22, 1–10 (2022). https://doi.org/10.1186/s12906-022-03780-1
H.W. Choi, P.G. Shin, J.H. Lee et al., Anti-inflammatory effect of lovastatin is mediated via the modulation of NF-κB and inhibition of HDAC1 and the PI3K/Akt/mTOR pathway in RAW264.7 macrophages. Int J Mol Med 41, 1103–1109 (2018). https://doi.org/10.3892/ijmm.2017.3309
Y.N. Huang, C.C. Lai, C.T. Chiu et al., L-ascorbate attenuates the endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation and NF- κB translocation in cortical neurons/glia cocultures. PLoS ONE (2014). https://doi.org/10.1371/journal.pone.0097276
C.R. Jack, M.S. Albert, D.S. Knopman et al., Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7, 257–262 (2011). https://doi.org/10.1016/j.jalz.2011.03.004
S.Y. Park, E.H. Yi, Y. Kim, G. Park, Anti-neuroinflammatory effects of ephedra sinica stapf extract-capped gold nanoparticles in microglia. Int J Nanomed 14, 2861–2877 (2019). https://doi.org/10.2147/IJN.S195218
E. Candelario-Jalil, R.S. Akundi, H.S. Bhatia et al., Ascorbic acid enhances the inhibitory effect of aspirin on neuronal cyclooxygenase-2-mediated prostaglandin E2 production. J Neuroimmunol 174, 39–51 (2006). https://doi.org/10.1016/j.jneuroim.2006.01.003
P. Umarao, S. Bose, S. Bhattacharyya et al., Neuroprotective potential of superparamagnetic iron oxide nanoparticles along with exposure to electromagnetic field in 6-OHDA rat model of Parkinson’s disease. J Nanosci Nanotechnol 16, 261–269 (2016). https://doi.org/10.1166/jnn.2016.11103
S. Ballaz, I. Morales, M. Rodríguez, J.A. Obeso, Ascorbate prevents cell death from prolonged exposure to glutamate in an in vitro model of human dopaminergic neurons. J Neurosci Res 91, 1609–1617 (2013). https://doi.org/10.1002/jnr.23276
N. Zhang, W. Zhao, Z.J. Hu et al., Protective effects and mechanisms of high-dose vitamin C on sepsis-associated cognitive impairment in rats. Sci Rep 11, 1–10 (2021). https://doi.org/10.1038/s41598-021-93861-x
F. De Nuccio, A. Cianciulli, C. Porro et al., Inflammatory response modulation by vitamin c in an mptp mouse model of parkinson’s disease. Biology (Basel) (2021). https://doi.org/10.3390/biology10111155
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Luo, P., Wu, S. et al. Deciphering the neuroprotective effect of ascorbic acid mediated synthesis of iron oxide nanoparticles against Parkinson’s disease: an in vitro and in vivo approach. Macromol. Res. 31, 949–960 (2023). https://doi.org/10.1007/s13233-023-00186-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-023-00186-x