Abstract
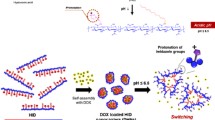
In this study, doxorubicin (DOX)-induced cardiotoxicity was overcome using cancer-recognizable DOX-loaded acetylated hyaluronic aicd-poly(L-lactic acid) (AcHA-PLLA) nanoparticles (DHPs). AcHA-PLLA was synthesized via ring opening polymerization of L-lactide with AcHA as a macroinitiator and characterized by 1H NMR and critical micelle concentration. DHPs were formed by self-assembly via dialysis, were estimated to be 160.7 ± 2.7 nm in average diameter, exhibited a slow rate release profile, and prevented DOX from being degraded in the circulation. In addition, DHPs demonstrated efficient cellular uptake into a colorectal cancer cell line (HCT-116) by CD44 receptor-mediated endocytosis, which was confirmed by in vitro cellular uptake, the competitive inhibition assay with free HA and flow cytometry. Furthermore, DHPs exhibited therapeutic efficacy against HCT-116 cells through receptor-mediated endocytosis: DHPs with free hyaluronic acid (HA) demonstrated 2-fold higher cell viability than DHPs without free HA. DHPs also inhibited doxorubicinol production, which induces cardiotoxicity, from DOX in vivo. The area-under-the-curve (AUC) of DOX administered as free DOX was 109.0 ± 25.0 µg min mL−1 and that from DHPs was 97.2 ± 10.2 µg min mL−1. Although these are comparable, the AUC of doxorubicinol was 1.36 ± 0.18 µg min mL−1 and 0.45 ± 0.12 µg min mL−1, with the treatment of free DOX-HCl and DHPs, respectively, demonstrating the reduction of the metabolic conversion of DOX to doxorubicinol in plasma. These results indicate the role of DHPs in preventing DOX transformation into doxorubicinol, which may contribute to the reduced toxicity. DHPs are promising cancer therapeutic nanoparticles, because they are highly biocompatible and tumor-recognizable, and prevent the DOX transformation..

Similar content being viewed by others
References
W. Park, S.-J. Park, and K. Na, Colloids Surf. B: Biointerf., 79, 501 (2010).
E. H. Seo, C. S. Lee, and K. Na, Adv. Healthcare Mater., 4, 2822 (2015).
H. Park, W. Park, and K. Na, Biomaterials, 35, 7963 (2014).
A. M. Rahman, S. W. Yusuf, and M. S. Ewer, Int. J. Nanomed, 2, 567 (2007).
Y. C. Barenholz, J. Control Release, 160, 117 (2012).
Y. Li, R. Liu, J. Yang, Y. Shi, G. Ma, Z. Zhang, and X. Zhang, Biomaterials, 41, 1 (2015).
C.-S. Lee and K. Na, Biomacromolecules, 15, 4228 (2014).
W. Park, B.-C. Bae, and K. Na, Biomaterials, 77, 227 (2016).
K. S. Kim, W. Park, and K. Na, Biomaterials, 36, 90 (2015).
F. Li and K. Na, Biomacromolecules, 12, 1724 (2011).
K. Kim, C.-S. Lee, and K. Na, Chem. Commun., 52, 2839 (2016).
J. Seo, J. Lee, C. B. Lee, S. K. Bae, and K. Na, Bioconjugate Chem., 30, 621 (2019).
Z.-Y. Zhou, L.-L. Wan, Q.-J. Yang, Y.-L. Han, Y. Li, Q. Yu, C. Guo, and X. Li, Toxicol. Appl. Pharmacol., 272, 238 (2013).
S. Seo, C.-S. Lee, Y.-S. Jung, and K. Na, Carbohydr. Polym, 87, 1105 (2012).
J. K. Cho, W. Park, and K. Na, J. Appl Polym Sci., 113, 2209 (2009).
F. Li, B.-C. Bae, and K. Na, Bioconjugate Chem, 21, 1312 (2010).
S. Acharya and S. K. Sahoo, Adv. Drug Deliv. Rev., 63, 170 (2011).
H. S. S. Qhattal and X. Liu, Mol. Pharmaceut, 8, 1233 (2011).
T. Fujisaki, Y. Tanaka, K. Fujii, S. Mine, K. Saito, S. Yamada, U. Yamashita, T. Irimura, and S. Eto, Cancer Res., 59, 4427 (1999).
E.-K. Lim, H.-O. Kim, E. Jang, J. Park, K. Lee, J.-S. Suh, Y.-M. Huh, and S. Haam, Biomaterials, 32, 7941 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments: This work was supported by Basic Research Laboratory (BRL) Program (NRF-2015R1A4A1042350) and Nano-Material Technology Development Program (NRF-2018M3A7B4071235) Through the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (MSIT) and research funds from the Catholic University of Korea (Research Fund 2019).
Supporting Information
Rights and permissions
About this article
Cite this article
Jo, Yu., Lee, C.B., Bae, S.K. et al. Acetylated Hyaluronic Acid-Poly(L-lactic acid) Conjugate Nanoparticles for Inhibition of Doxorubicinol Production from Doxorubicin. Macromol. Res. 28, 67–73 (2020). https://doi.org/10.1007/s13233-020-8003-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-020-8003-6