Abstract
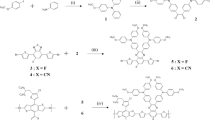
Three quinoxaline-based small molecules possessing multiple electron-withdrawing moieties were synthesized by the Suzuki coupling reaction for organic photovoltaic cells (OPVs). The electron-donating triarylamine units were linked to both ends of electron-accepting 2,3-diphenyl quinoxaline (DPQ) derivatives with strong electron-withdrawing trifluoromethyl (CF3) moieties to produce a reference D-A-D type small molecule of CF3Qx-0F. Furthermore, one and two fluorine atoms were additionally introduced to the 6,7-positions ofthe DPQ unit of CF3Qx-0F affording CF3Qx-1F and CF3Qx-2F, respectively. Owingto the significant contribution of the electron-withdrawing CF3 and fluorine units, all inverted-type OPVs based on three small molecules exhibited high open circuit voltages greater than 0.82 V. In addition, the power conversion efficiencies (PCEs) of the devices were gradually improved with increasing number of fluorine atoms. The highest PCE (2.82%) with a Voc of 0.88 V, a short-circuit current of 6.38 mA cm−2, and a fill factor of 50.6% was achieved from the device based on CF3Qx-2F.

Similar content being viewed by others
References
Y. Chen, X. Wan, and G. Long, Acc. Chem. Res., 46, 2645 (2013).
B. Walker, C. Kim, and T.-Q. Nguyen, Chem. Mater., 23, 470 (2010).
H. Bin, J. Yao, Y. Yang, I. Angunawela, C. Sun, L. Gao, L. Ye, B. Qiu, L. Xue, and C. Zhu, Adv. Mater., 30, 1706361 (2018).
Y. Lin, Y. Li, and X. Zhan, Chem. Soc. Rev., 41, 4245 (2012).
Y. Huo, X.-T. Gong, T.-K. Lau, T. Xiao, C. Yan, X. Lu, G. Lu, X. Zhan, and H.-L. Zhang, Chem. Mater., 30, 8661 (2018).
R. Ilmi, A. Haque, and M. Khan, Org. Electron., 58, 53 (2018).
S. E-Ela, H. Chen, A. Kratzer, A. Hirsch, and C. J. Brabec, New J. Chem., 40, 2829 (2016).
D. Baran, S. E-Ela, A. Kratzer, T. Ameri, C. J. Brabec, and A. Hirsch, RSC Adv., 79, 64724 (2015).
S. E-Ela, C. Vilegas, J. L. Delgado, and N. Martin, New J. Chem., 39, 1477 (2015).
S. D. Collins, N. A. Ran, M. C. Heiber, and T. Q. Nguyen, Adv. Energy Mater., 7, 1602242 (2017).
J. Wan, X. Xu, G. Zhang, Y. Li, K. Feng, and Q. Peng, Energ. Environ. Sci., 10, 1739 (2017).
A. C. Stuart, J. R. Tumbleston, H. Zhou, W. Li, S. Liu, H. Ade, and W. You, J. Am. Chem. Soc., 135, 1806 (2013).
X.-P. Xu, Y. Li, M.-M. Luo, and Q. Peng, Chin. Chem Lett, 27, 1241 (2016).
J. Y. Shim, T. Kim, J. Kim, J. Kim, I. Kim, J. Y. Kim, and H. Suh, Synth Met., 205, 112 (2015).
J. Yuan, L. Qiu, Z.-G. Zhang, Y. Li, Y. Chen, and Y. Zou, Nano Energ., 30, 312 (2016).
A. Casey, S. D. Dimitrov, P. Shakya-Tuladhar, Z. Fei, M. Nguyen, Y. Han, T. D. Anthopoulos, J. R. Durrant, and M. Heeney, Chem. Mater., 28, 5110 (2016).
S. K. Putri, Y. H. Kim, D. R. Whang, M. S. Lee, J. H. Kim, and D. W. Chang, Org. Electron., 47, 14 (2017).
S. Xu, L. Feng, J. Yuan, Z.-G. Zhang, Y. Li, H. Peng, and Y. Zou, ACS Appl. Mater. Inter., 9, 18816 (2017).
M. Li, G. Zhang, L. Xiong, M. Zhu, Y. Pei, Q. Peng, and Y. Liu, Dyes Pigments, 158, 402 (2018).
N. D. Eastham, A. S. Dudnik, B. Harutyunyan, T. J. Aldrich, M. J. Leonardi, E. F. Manley, M. R. Butler, T. Harschneck, M. A. Ratner, and L. X. Chen, ACS Energy Lett, 2, 1690 (2017).
L. Liao, L. Dai, A. Smith, M. Durstock, J. Lu, J. Ding, and Y. Tao, Macromolecules, 40, 9406 (2007).
J. Kim, M. H. Yun, G.-H. Kim, J. Lee, S. M. Lee, S.-J. Ko, Y. Kim, G. K. Dutta, M. Moon, and S. Y. Park, ACS Appl. Mater. Inter., 6, 7523 (2014).
S. K. Putri, Y. H. Kim, D. R Whang, J. H. Kim, and D. W. Chang, Macromol. Rapid Commun., 39, 1800260 (2018).
B. Liang, L. Wang, Y. Xu, H. Shi, and Y. Cao, Adv. Funct Mater., 17, 3580 (2007).
S. K. Putri, M. S. Lee, D. W. Chang, and J. H. Kim, Synth Met, 220, 455 (2016).
S. Song, T. Kim, H. Park, Y. Jin, I. Kim, J. Y. Kim, and H. Suh, Synth Met, 183, 16 (2013).
M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. Petersson, Gaussian 09, revision b. 01, Gaussian, Inc., Wallingford, CT, 6492 (2010).
S. Yum, T. K. An, X. Wang, W. Lee, M. A. Uddin, Y. J. Kim, T. L. Nguyen, S. Xu, S. Hwang, and C. E. Park, Chem. Mater., 26, 2147 (2014).
Y. Li, T. H. Lee, S. Y. Park, M. A. Uddin, T. Kim, S. Hwang, J. Y. Kim, and H. Y. Woo, Polym Chem., 7, 4638 (2016).
J. Xue, S. Uchida, B. P. Rand, and S. R. Forrest, Appl. Phys. Lett, 85, 5757 (2004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnote: The image from this article is used as the cover image of the Volume 27, Issue 12.
Acknowledgment: This research was supported by Basic Science Researches through the National Research Foundation (NRF) of Korea under program number (2018R1D1A1B07042822 and 2016R1D1A1B03930154).
Supporting Information
Rights and permissions
About this article
Cite this article
Kim, J.T., Jin, H.C., Putri, S.K. et al. Synthesis of Quinoxaline-Based Small Molecules Possessing Multiple Electron-Withdrawing Moieties for Photovoltaic Applications. Macromol. Res. 27, 1268–1274 (2019). https://doi.org/10.1007/s13233-020-8002-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-020-8002-7