Abstract
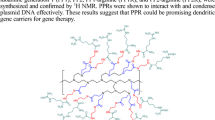
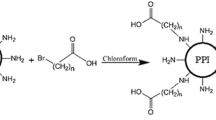
Poly(amidoamine) (PAMAM) dendrimers are synthetic polymers commonly used as carriers in gene and drug delivery. PAMAMs have the ability to transfect DNA, but their transfection efficiency is currently insufficient for clinical use. Here, we demonstrate the synthesis and evaluation of cationic dendrimers consisting of a cystamine core PAMAM generation 3 (cPAM G3) and amino acids. Introduction of histidine (His) and arginine (Arg) residues to cPAM G3 resulted in high transfection efficiency and low cytotoxicity. cPAM G3-His-Arg formed stable polyplexes at a weight ratio of 6:1, and the mean polyplex diameter was 99.03±1.68 nm. Nano-sized polyplexes increased in diameter up to 132.93±1.79 nm when treated with a reducing agent, dithiothreitol (DTT). cPAM G3-His-Arg showed much higher transfection efficiency than native cPAM G3 and polyethylenimine (PEI, 25KD). In addition, cPAM G3-His-Arg displayed negligible toxicity, even at high polymer concentrations. Finally, confocal laser microscopy results showed that cPAM G3-His-Arg effectively internalized plasmid DNA into the cells. Therefore, we believe that cPAM G3-His-Arg could be a promising bioreducible vector for non-viral gene delivery with high gene transfection efficiency and low cytotoxicity.

Similar content being viewed by others
References
D. A. Tomalia and J. M. J. Frechet, J. Polym. Sci. Part A: Polym. Chem., 40, 2719 (2002).
A. Tomalia D., H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, and P. Smith, Polym. J., 17, 177 (1985).
M. J. Cloninger, Curr. Opin. Chem. Biol., 6, 742 (2002).
C. C. Lee, J. A. MacKay, J. M. J. Frechet, and F. C. Szoka, Nat. Biotechnol., 23, 1517 (2005).
V. J. Venditto, C. A. S. Regino, and M. W. Brechbiel, Mol. Pharm., 2, 302 (2005).
G. Y. Wu and C. H. Wu, J. Biol. Chem., 262, 4429 (1987).
O. Boussif, F. Lezoualch, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix, and J. P. Behr, Proc. Natl. Acad. Sci. U.S.A., 92, 7297 (1995).
C. W. Pouton and L. W. Seymour, Adv. Drug Deliv. Rev., 46, 187 (2001).
G. D. Schmidt-Wolf and I. G. H. Schmidt-Wolf, Trends Mol. Med., 9, 67 (2003).
J. S. Choi, K. Nam, J. Y. Park, J. B. Kim, J. K. Lee, and J. S. Park, J. Control. Release, 99, 445 (2004).
K. Kono, H. Akiyama, T. Takahashi, T. Takagishi, and A. Harada, Bioconjug. Chem., 16, 208 (2005).
R. Shukla, T. P. Thomas, J. Peters, A. Kotlyar, A. Myc, and Jr. J. R. Baker, Chem. Commun., 5739 (2005).
D. Luo, K. Haverstick, N. Belcheva, E. Han, and W. M. Saltzman, Macromolecules, 35, 3456 (2002).
T. Merdan, K. Kunath, H. Petersen, U. Bakowsky, K. H. Voigt, J. Kopecek, and T. Kissel, Bioconjug. Chem., 16, 785 (2005).
R. B. Kolhatkar, K. M. Kitchens, P. W. Swaan, and H. Ghandehari, Bioconjug. Chem., 18, 2054 (2007).
G. S. Yu, Y. M. Bae, H. Choi, B. Kong, I. S. Choi, and J. S. Choi, Bioconjug. Chem., 22, 1046 (2011).
M. A. Gosselin, W. J. Guo, and R. J. Lee, Bioconjug. Chem., 12, 989 (2001).
K. Miyata, Y. Kakizawa, N. Nishiyama, A. Harada, Y. Yamasaki, H. Koyama, and K. Kataoka, J. Am. Chem. Soc., 126, 2355 (2004).
T. I. Kim, M. Ou, M. Lee, and S. W. Kim, Biomaterials, 30, 658 (2009).
Q. Peng, Z. L. Zhong, and R. X. Zhuo, Bioconjug. Chem., 19, 499 (2008).
Y. Lee, H. Mo, H. Koo, J. Y. Park, M. Y. Cho, G. W. Jin, and J. S. Park, Bioconjug. Chem., 18, 13 (2007).
H. Mok and T. G. Park, Biopolymers, 89, 881 (2008).
Y. W. Won, H. A. Kim, M. Lee, and Y. H. Kim, Mol. Ther., 18, 734 (2010).
Y. Kakizawa, A. Harada, and K. Kataoka, Biomacromolecules, 2, 491 (2001).
H. Mok, J. W. Park, and T. G. Park, Bioconjug. Chem., 18, 1483 (2007).
C. Lin, Z. Y. Zhong, M. C. Lok, X. L. Jiang, W. E. Hennink, J. Feijen, and J. F. J. Engbersen, Bioconjug. Chem., 18, 138 (2007).
S. M. Moghimi, P. Symonds, J. C. Murray, A. C. Hunter, G. Debska, and A. Szewczyk, Mol. Ther., 11, 990 (2005).
Author information
Authors and Affiliations
Corresponding authors
Additional information
The first two authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, G.S., Bae, Y.M., Kim, J.Y. et al. Amino acid-modified bioreducible poly(amidoamine) dendrimers: Synthesis, characterization and In vitro evaluation. Macromol. Res. 20, 1156–1162 (2012). https://doi.org/10.1007/s13233-012-0164-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-012-0164-5