Abstract
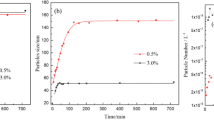
The reversible iodine transfer polymerization (RITP) of styrene in the absence of light was carried out using potassium persulfate (KPS) under argon atmosphere at 80 °C for 7 h and the properties of the polymers were compared to those obtained from the soap-free emulsion polymerization (SFEP). The variables were the concentration of KPS and iodine, molar ratios between KPS and iodine, reaction temperatures, and monomer contents. The results showed that the conversion retarded and reached from 99.5% to 82%, the particle size dramatically increased from 0.335 to 1.37 μm, the molecular weight decreased from 183,000 to 66,000 g/mol, and the polydispersity index (PDI) increased from 2.7 to 3.8 for the presence of 2 wt% of KPS and iodine content between 0 and 0.228 mmol, respectively, implying that the molecular weight was controlled and that the particle stability was reduced with iodine as usual. For the effects of the solid content on particle properties, the monomer conversion and the particle size increased, but the molecular weight and PDI decreased with the increased KPS content and [KPS]/[I2] ratio. The highest content was 30 wt% and this was less than that in the presence of sodium dodecyl sulfate (SDS) in the RITP-emulsion and in the absence of SDS in the SFEP. Thus, the use of an emulsifier in the RITP or SFEP was one of the important factors for obtaining the maximum solid content needed for industrial applications.

Similar content being viewed by others
References
R. G. Gilbert, Emulsion Polymerization: A Mechanistic Approach, Academic Press, London, 1995.
X. Li, M. Huang, J. Zeng, and M. Zhu, Colloids Surf. A: Physicochem. Eng. Asp., 248, 111 (2004).
X. Li, H. Zhou, and M. Huang, Polymer, 46, 1523 (2005).
E. S. Daniels, E. D. Sudol, and M. S. El-Aasser, ACS Symp. Ser., 492, 1 (1992).
J. S. Guo, M. S. El-Aasser, and J. W. Vanderhoff, J. Polym. Sci. Part A: Polym. Chem., 27, 691 (1989).
K. Matyjaszewski and A. H. E. Mueller, Polymer Prepr., 38, 6 (1997).
K. Matyjaszewski, ACS Symp. Ser., 768, 1 (2000).
J. Tonnar and P. Lacroix-Desmazes, Angew. Chem., 120, 1314 (2008).
B. Bailly, A. Donnenwirth, C. Bartholome, E. Beyou, and E. Bourgeat-Lami, J. Nanomater., 2006, 1 (2006).
R. W. Simms and M. F. Cunningham, Macromol. Symp., 261, 32 (2008).
M. C. Iovu and K. Matyjaszewski, Macromolecules, 36, 9346 (2003).
J. Chiefari, R. T. A. Mayadunne, C. L. Moad, G. Moad, E. Rizzardo, A. Postma, M. A. Skidmore, and S. H. Thang, Macromolecules, 36, 2273 (2003).
H. C. Shin, H. G. Oh, K. Lee, B. H. Lee, and S. Choe, Polymer, 50, 4299 (2009).
P. Lacroix-Desmazes, R. Severac, and B. Boutevin, Macromolecules, 38, 6299 (2005).
G. David, C. Boyer, J. Tonnar, B. Ameduri, P. Lacroix-Desmazes, and B. Boutevin, Chem. Rev., 106, 3936 (2006).
D. S. Trifan and P. D. Bartlett, J. Am. Chem. Soc., 81, 5573 (1959).
P. Ghosh, A. N. Banerjee, P. S. Mitra, and S. Chakraborty, J. Polym. Sci. Polym. Lett. Ed., 13, 35 (1975).
J. Tonnar, P. Lacroix-Desmazes, and B. Boutevin, Macromolecules, 40, 186 (2007).
H. G. Oh, H. C. Shin, H. Jung, B. H. Lee, and S. Choe, J. Colloid Interface Sci., 353, 459 (2011).
C. S. Chern, Prog. Polym. Sci. (Oxford), 31, 443 (2006).
W. Chiu and C. Shih, J. Appl. Polym. Sci., 31, 2117 (1986).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y., Kim, K., Lee, B.H. et al. Molecular weight control of PS spheres using soap free and RITP-soap free emulsion polymerization. Macromol. Res. 20, 977–984 (2012). https://doi.org/10.1007/s13233-012-0144-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-012-0144-9