Abstract
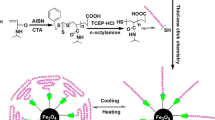
Amphiphilic block copolymers were synthesized by reversible addition fragmentation chain transfer (RAFT) radical block polymerization of N-vinylimidazole and 3-(methacrylamido)phenylboronic acid using poly (ethylene oxide) (PEO)-based xanthate type of RAFT agent and 2,2′-azobisisobutyronitrile (AIBN) in dimethylformaide (DMF) at 90∼110 °C for 24 h. Poly(ethylene oxide-b-3-(methacrylamido)phenylboronic acid) and poly(ethylene oxide-b-N-vinylimidazole-b-3-(methacrylamido)phenylboronic acid) were prepared successfully through the sequential monomer addition method. Both block copolymers were found to exhibit glucose-responsive behavior via the feasibility test for complex formation with Alizarin Red S (ARS). The ratio of molecular weight of the poly(N-vinyimidazole) to the poly(3-(methacrylamido)phenylboronic acid) appeared to play an important role in the pH- & glucose-responsive behavior. The resulting block copolymers were used successfully as polymeric stabilizers to prepare water-soluble Fe3O4 nanoparticles. All the materials were characterized by the combination of 1H nuclear magnetic resonance (NMR), size exclusion chromatography (SEC), transmission electron microscopy (TEM), thermogravimetric analysis (TGA), and x-ray diffraction (XRD) analyses.
Similar content being viewed by others
References
I. C. Kwon, Y. H. Bae, and S. W. Kim, Nature, 354, 291 (1991).
A. S. Hoffmann, in Controlled Drug Delivery: Challenges and Strategies, K. N. Park, Ed., Am. Chem. Soc., Washington, D. C., 1997, Chap. 24, pp 485–498.
T. D. James, S. K. R. A. Sandanayake, and S. Shinkai, Angew. Chem. Int. Eng. 35, 1910 (1996)
T. D. James and S. Shinkai, Top. Curr. Chem., 218, 159 (2002)
Z. M. O. Rzayev and O. Beskardes, Coll. Czech. Chem. Commun., 72, 1591 (2007).
T. D. James, K. R. A. S. Sandanayake, and S. Shinkai, Nature, 374, 345 (1995).
M. L. Stolowitz, C. Ahlem, K. A. Hughes, R. J. Kaiser, E. A. Kesicki, G. Li, K. P. Lund, S. M. Torkelson, and J. P. Wiley, Bioconjug. Chem., 12, 229 (2001).
N. Di Cesare and J. R. Lakowicz, J. Phys. Chem. A, 105, 6834 (2001).
X. Jin, X. Zhang, Z. Wu, D. Teng, X. Zhang, Y. Wang, Z. Wang, and C. Li, Biomacromolecules, 10, 1337 (2009).
G. Achanta, A. Modzelewska, L. Feng, S. R. Khan, and P. Huang, Mol. Pharmacol., 70, 426 (2006).
H. J. Jeon, D. H. Go, S. Choi, K. M. Kim, J. Y. Lee, D. J. Choo, H.-O. Yoo, J. M. Kim, and J. Kim, Colloid Surf. A: Physicochem. Eng. Asp., 317, 496 (2008)
K. Kim, T. H. Kim, J. H. Choi, J. Y. Lee, S. S. Hah, H.-O. Yoo, S. S. Hwang, K. N. Ryu, H. J. Kim, and J. Kim, Macromol. Chem. Phys., 211, 1127 (2010).
J. Kim, S. Choi, K. M. Kim, D. H. Go, H. J. Jeon, J. Y. Lee, H. S. Park, C. H. Lee, and H. M. Park, Macromol. Res., 15, 337 (2007).
J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. O. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo, and S. H. Thang, Macromolecules, 31, 5559 (1998)
M. Destarac, D. Charmot, X. Franck, and S. Z. Zard, Macromol. Rapid Commun., 21, 1035 (2000)
K. Matyaszewski, in Handbook of Radical Polymerization, K. Matyaszewski and T. P. Davis, Eds., John Wiley & Sons, Inc., 2002, Chap. 8, pp 361–406.
T. Hoare and R. Pelton, Biomacromolecules, 9, 733 (2008)
D. Roy, J. N. Cambre, and B. S. Sumerlin, Chem. Commun., 2106 (2009)
P. A. Sienkiewicz and D. C. Roberts, J. Inorg. Nucl. Chem., 42, 1559 (1980)
X. Yang, M.-C. Lee, F. Sartain, X. Pan, and C. R. Lowe, Chem. Eur. J., 12, 8491 (2006).
M. Oishi, S. Sumitani, and Y. Nanasaki, Bioconjug. Chem., 18, 1379 (2007).
D. Roy, J. N. Cambre, and B. S. Sumerlin, Chem. Commun., 2477 (2008)
A. Matsumoto, K. Yamamoto, R. Yoshida, K. Kataoka, T. Aoyagi, and Y. Miyahara, Chem. Commun., 46, 2203 (2010)
K. Shiomori, A. E. Ivanov, I. Yu, Y. Kawano, and B. Mattiasson, Macromol. Chem. Phys., 205, 27 (2004)
A. Ivanov, I. Yu, and B. Mattiasson, J. Mol. Recogn., 19, 322 (2006).
J. Yoon and A. W. Czarnik, J. Am. Chem. Soc., 114, 5874 (1992)
G. Springsteen and B. Wang, Chem. Commun., 1608 (2001)
W. Wang, X. Gao, and B. Wang, Curr. Org. Chem., 6, 1285 (2002)
H. Fang, G. Kaur, and B. Wang, J. Fluores., 14, 481 (2004)
J. Yan, H. Fang, and B. Wang, Med. Res. Rev., 25, 490 (2005).
L. Zhu, S. H. Shabbir, M. Gray, V. M. Lynch, S. Sorey, and E. V. Anslyn, J. Am. Chem. Soc., 128, 1222 (2006)
W. Chen, R. Pelton, and V. Leung, Macromolecules, 42, 1300 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, S., Nam, J.H., Kim, Y.J. et al. Synthesis of PEO-based glucose-sensitive block copolymers and their application for preparation of superparamagnetic iron oxide nanoparticles. Macromol. Res. 19, 827–834 (2011). https://doi.org/10.1007/s13233-011-0810-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-011-0810-3