Abstract
Freshwater fungi comprises a highly diverse group of organisms occurring in freshwater habitats throughout the world. During a survey of freshwater fungi on submerged wood in streams and lakes, a wide range of sexual and asexual species were collected mainly from karst regions in China and Thailand. Phylogenetic inferences using partial gene regions of LSU, ITS, SSU, TEF1α, and RPB2 sequences revealed that most of these fungi belonged to Dothideomycetes and Sordariomycetes and a few were related to Eurotiomycetes. Based on the morphology and multi-gene phylogeny, we introduce four new genera, viz. Aquabispora, Neocirrenalia, Ocellisimilis and Uvarisporella, and 47 new species, viz. Acrodictys chishuiensis, A. effusa, A. pyriformis, Actinocladium aquaticum, Annulatascus tratensis, Aquabispora setosa, Aqualignicola setosa, Aquimassariosphaeria vermiformis, Ceratosphaeria flava, Chaetosphaeria polygonalis, Conlarium muriforme, Digitodesmium chishuiense, Ellisembia aquirostrata, Fuscosporella atrobrunnea, Halobyssothecium aquifusiforme, H. caohaiense, Hongkongmyces aquisetosus, Kirschsteiniothelia dushanensis, Monilochaetes alsophilae, Mycoenterolobium macrosporum, Myrmecridium splendidum, Neohelicascus griseoflavus, Neohelicomyces denticulatus, Neohelicosporium fluviatile, Neokalmusia aquibrunnea, Neomassariosphaeria aquimucosa, Neomyrmecridium naviculare, Neospadicoides biseptata, Ocellisimilis clavata, Ophioceras thailandense, Paragaeumannomyces aquaticus, Phialoturbella aquilunata, Pleurohelicosporium hyalinum, Pseudodactylaria denticulata, P. longidenticulata, P. uniseptata, Pseudohalonectria aurantiaca, Rhamphoriopsis aquimicrospora, Setoseptoria bambusae, Shrungabeeja fluviatilis, Sporidesmium tratense, S. versicolor, Sporoschisma atroviride, Stanjehughesia aquatica, Thysanorea amniculi, Uvarisporella aquatica and Xylolentia aseptata, with an illustrated account, discussion of their taxonomic placement and comparison with morphological similar taxa. Seven new combinations are introduced, viz. Aquabispora grandispora (≡ Boerlagiomyces grandisporus), A. websteri (≡ Boerlagiomyces websteri), Ceratosphaeria suthepensis (≡ Pseudohalonectria suthepensis), Gamsomyces aquaticus (≡ Pseudobactrodesmium aquaticum), G. malabaricus (≡ Gangliostilbe malabarica), Neocirrenalia nigrospora (≡ Cirrenalia nigrospora), and Rhamphoriopsis glauca (≡ Chloridium glaucum). Ten new geographical records are reported in China and Thailand and nine species are first reported from freshwater habitats. Reference specimens are provided for Diplocladiella scalaroides and Neocirrenalia nigrospora (≡ Cirrenalia nigrospora). Systematic placement of the previously introduced genera Actinocladium, Aqualignicola, and Diplocladiella is first elucidated based on the reference specimens and new collections. Species recollected from China and Thailand are also described and illustrated. The overall trees of freshwater Dothideomycetes and Sordariomycetes collected in this study are provided respectively and genera or family/order trees are constructed for selected taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Table of contents
Ascomycota Caval.-Sm
Dothideomycetes O.E. Erikss & Winka
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss.
Stictographaceae D.Q. Dai & K.D. Hyde
1. Actinocladium aquaticum J. Yang & K.D. Hyde, sp. nov.
Kirschsteiniotheliales Hern.-Restr., R.F. Castañeda, Gené & Crous
Kirschsteiniotheliaceae Boonmee & K.D. Hyde
2. Kirschsteiniothelia dushanensis L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
Natipusillales Raja, Shearer, A.N. Mill. & K.D. Hyde
Natipusillaceae Raja, Shearer & A.N. Mill
3. Natipusilla limonensis A. Ferrer, A.N. Mill. & Shearer, new record in Thailand
Pleosporales Luttr. ex M.E. Barr
Amniculicolaceae Yin. Zhang, C.L. Schoch, J. Fourn., Crous & K.D. Hyde
4. Amniculicola asexualis Magaña-Dueñas, Stchigel & Cano, new record in China
5. Neomassariosphaeria aquimucosa L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
Aquasubmersaceae A. Hashim. & Kaz. Tanaka
6. Aquasubmersa japonica A. Hashim. & Kaz. Tanaka, new record in Thailand
Astrosphaeriellaceae Phookamsak & K.D. Hyde
7. Caryospora aquatica H. Zhang, K.D. Hyde & Ariyawansa, recollected in China
8. Pithomyces flavus Berk. & Broome, recollected in Thailand
Dictyosporiaceae Boonmee & K.D. Hyde
9. Digitodesmium chishuiense J. Yang & K.D. Hyde, sp. nov.
Didymosphaeriaceae Munk
10. Austropleospora ochracea L.S. Dissan, J.C. Kang & K.D. Hyde, new habitat, recollected in China
11. Neokalmusia aquibrunnea J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Lentitheciaceae Yin. Zhang, C.L. Schoch, J. Fourn., Crous & K.D. Hyde
12. Halobyssothecium aquifusiforme J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
13. Halobyssothecium caohaiense L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
14. Lentithecium pseudoclioninum Kaz. Tanaka & K. Hiray., new record in China
15. Setoseptoria bambusae J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
16. Towyspora aestuari Wanasinghe, E.B.G. Jones & K.D. Hyde, new record in China
Lindgomycetaceae K. Hiray., Kaz. Tanaka & Shearer
17. Aquimassariosphaeria vermiformis L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
18. Hongkongmyces aquisetosus J. Yang Jian K. Liu & K.D. Hyde, sp. nov.
19. Ocellisimilis J. Yang, L.L. Liu & K.D. Hyde, gen. nov.
20. Ocellisimilis clavata L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
Longipedicellataceae Phukhams., J. Bhat & K.D. Hyde
21. Longipedicellata aquatica W. Dong, H. Zhang & K.D. Hyde, recollected in Thailand
22. Pseudoxylomyces elegans (Goh, W.H. Ho, K.D. Hyde & C.K.M. Tsui) Kaz. Tanaka & K. Hiray., recollected in Thailand
Morosphaeriaceae Suetrong, Sakayaroj, E.B.G. Jones & C.L. Schoch
23. Aquihelicascus thalassioideus (K.D. Hyde & Aptroot) W. Dong & H. Zhang, recollected in Thailand
24. Neohelicascus chiangraiensis (Z.L. Luo, J.K. Liu, H.Y. Su & K.D. Hyde) W. Dong, K.D. Hyde & H. Zhang, recollected in China
25. Neohelicascus gallicus (Y. Zhang & J. Fourn) W. Dong, K.D. Hyde & H. Zhang, new record in China and Thailand
26. Neohelicascus griseoflavus J. Yang & K.D. Hyde, sp. nov.
Parabambusicolaceae Kaz. Tanaka & K. Hiray.
27. Parabambusicola bambusina (Teng) Kaz. Tanaka & K. Hiray., new habitat, recollected in China
Phaeoseptaceae S. Boonmee, Thambugala & K.D. Hyde
28. Pleopunctum ellipsoideum N.G. Liu, K.D. Hyde & J.K. Liu, new habitat, recollected in China
Pleosporaceae Nitschke
29. Alternaria scirpivora (E.G. Simmons & D.A. Johnson) Woudenb. & Crous, recollected in China
Pseudoastrosphaeriellaceae Phookamsak & K.D. Hyde
30. Pseudoastrosphaeriella bambusae Phookamsak & K.D. Hyde, new record in China
Testudinaceae Arx
31. Mycoenterolobium macrosporum J. Yang & K.D. Hyde, sp. nov.
Tetraplosphaeriaceae Kaz. Tanaka & K. Hiray.
32. Shrungabeeja fluviatilis J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Torulaceae Corda
33. Rostriconidium aquaticum Z.L. Luo, K.D. Hyde & H.Y. Su, recollected in China
Tubeufiales Boonmee & K.D. Hyde
Tubeufiaceae M.E. Barr
34. Helicoarctatus aquaticus Y.Z. Lu, J.C. Kang & K.D. Hyde, recollected in Thailand
35. Neohelicomyces denticulatus L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
36. Neohelicosporium fluviatile J. Yang & K.D. Hyde, sp. nov.
37. Pleurohelicosporium hyalinum J. Yang, Jian K. Liu & K.D. Hyde sp. nov.
Dothideomycetes genera incertae sedis
38. Diplocladiella scalaroides G. Arnaud ex M.B. Ellis, reference specimen, recollected in China
39. Oncopodiella trigonella (Sacc.) Rifai, new habitat, recollected in China
Eurotiomycetes O.E. Erikss. & Winka
Chaetothyriales M.E. Barr
Herpotrichiellaceae Munk
40. Thysanorea amniculi J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
41. Thysanorea nonramosa (X.D. Yu, G.N. Wang & H. Zhang) Hern.-Restr. & Crous, recollected in Thailand
42. Veronaea botryosa Cif. & Montemart., recollected in China
Sclerococcales Réblová, Unter. & W. Gams
Dactylosporaceae Bellem. & Hafellner
43. Gamsomyces aquaticus (W. Dong, H. Zhang & K.D. Hyde) J. Yang & K.D. Hyde, comb. nov.
44. Gamsomyces longisporus (M.B. Ellis) Hern.-Restr. & Réblová, recollected in China
45. Gamsomyces malabaricus (Subram. & Bhat) J. Yang & K.D. Hyde, comb. nov.
46. Gamsomyces stilboideus (R.F. Castañeda & G.R.W. Arnold) Hern.-Restr. & Réblová, recollected in Thailand
Sordariomycetes O.E. Erikss. & Winka
Amphisphaeriales D. Hawksw. & O.E. Erikss.
Amphisphaeriaceae G. Winter
47. Amphisphaeria uniseptata (C.K.M. Tsui, K.D. Hyde & Hodgkiss) Samarak., Maharachch. & K.D. Hyde, recollected in China
Annulatascales M.J. D'souza, Maharachch. & K.D. Hyde
Annulatascaceae S.W. Wong, K.D. Hyde & E.B.G. Jones
48. Annulatascus tratensis J. Yang & K.D. Hyde, sp. nov.
Atractosporales H. Zhang, K.D. Hyde & Maharachch.
Pseudoproboscisporaceae H. Zhang, K.D. Hyde & Maharachch.
49. Aqualignicola setosa J. Yang & K.D. Hyde, sp. nov.
Chaetosphaeriales Huhndorf, A.N. Mill. & F.A. Fernández
Chaetosphaeriaceae Réblová, M.E. Barr & Samuels
50. Chaetosphaeria polygonalis J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
51. Codinaea lignicola (Z.L. Luo, H.Y. Su & K.D. Hyde) Réblová & Hern.-Restr., recollected in China, morphology differs from the holotype
52. Ellisembia aquirostrata J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
53. Fuscocatenula variegata (H.H. Li & X.G. Zhang) Réblová & A.N. Mill., new habitat, recollected in China
54. Kionochaeta castaneae C.G. Lin & J.K. Liu, new habitat, recollected in China, morphology differs from the holotype
55. Menisporopsis dushanensis C.G. Lin & K.D. Hyde, new habitat, recollected in China
56. Neocirrenalia J. Yang & K.D. Hyde, gen. nov.
57. Neocirrenalia nigrospora (Somrith., Chatmala & E.B.G. Jones) J. Yang & K.D. Hyde, comb. nov., reference specimen, recollected in Thailand
58. Nimesporella riisgaardii W.P. Wu & Y.Z. Diao, new habitat, recollected in China
59. Oxenbollia lunatospora W.P. Wu & Y.Z. Diao, new habitat, recollected in China
60. Paragaeumannomyces aquaticus J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
61. Phialoturbella aquilunata J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
62. Sporoschisma atroviride J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
63. Tainosphaeriella aquatica (X.D. Yu, C.X. Li & H. Zhang) Réblová & Hern.-Restr., recollected in Thailand
Conioscyphales Réblová & Seifert
Conioscyphaceae Réblová & Seifert
64. Vanakripa chiangmaiensis X.G. Tian & Karun., recollected in Thailand
Conlariales K.D. Hyde & Hongsanan
Conlariaceae H. Zhang, K.D. Hyde & Maharachch.
65. Conlarium muriforme J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Fuscosporellales J. Yang, J. Bhat & K.D. Hyde
Fuscosporellaceae J. Yang, J. Bhat & K.D. Hyde
66. Fuscosporella atrobrunnea L.L. Liu, J. Yang & Z.Y. Liu, sp. nov.
Glomerellales Chadef. ex Réblová, W. Gams & Seifert
Australiascaceae Réblová & W. Gams
67. Monilochaetes alsophilae J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Reticulascaceae Réblová & W. Gams
68. Kylindria peruamazonensis Matsush., new record in Thailand
Hypocreales Lindau
Tilachlidiaceae L. Lombard & Crous
69. Uvarisporella J. Yang, Jian K. Liu & K.D. Hyde, gen. nov.
70. Uvarisporella aquatica J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Magnaporthales Thongk., Vijaykr. & K.D. Hyde
Ceratosphaeriaceae Z.L. Luo, H.Y. Su & K.D. Hyde
71. Ceratosphaeria flava J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
72. Ceratosphaeria suthepensis (I. Promputtha) J. Yang & K.D. Hyde, comb. nov.
Ophioceraceae Klaubauf, E.G. LeBrun & Crous
73. Ophioceras thailandense J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Pseudohalonectriaceae Hongsanan & K.D. Hyde.
74. Pseudohalonectria aurantiaca J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Myrmecridiales Crous
Myrmecridiaceae Crous
75. Myrmecridium splendidum L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
76. Neomyrmecridium naviculare J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Pleurotheciales Réblová & Seifert
Pleurotheciaceae Réblová & Seifert
77. Dematipyriforma aquilariae L. Y. Sun, Hai-Yan Li, Xiang Sun & L.D. Guo, new record in Thailand
Pseudodactylariales Crous
Pseudodactylariaceae Crous
78. Pseudodactylaria denticulata J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
79. Pseudodactylaria longidenticulata J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
80. Pseudodactylaria uniseptata J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Rhamphoriales K.D. Hyde & Hongsanan
Rhamphoriaceae Réblová
81. Rhamphoriopsis aquimicrospora J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
82. Rhamphoriopsis glauca (Ellis & Everh.) J. Yang, Jian K. Liu & K.D. Hyde, comb. nov.
83. Rhodoveronaea aquatica Z.L. Luo, K.D. Hyde & H.Y. Su, recollected in China, emend the measurement of the holotype
84. Xylolentia aseptata J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Savoryellales Boonyuen, Suetrong, Sivichai, K.L. Pang & E.B.G. Jones
Savoryellaceae Jaklitsch & Réblová
85. Aquabispora J. Yang, E.B.G. Jones & K.D. Hyde, gen. nov.
86. Aquabispora setosa J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
87. Aquabispora grandispora (S.J. Stanley & K.D. Hyde) J. Yang, E.B.G. Jones & K.D. Hyde, comb. nov.
88. Aquabispora websteri (Shearer & J.L. Crane) J. Yang, E.B.G. Jones & K.D. Hyde, comb. nov.
Sordariales Chadef. ex D. Hawksw. & O.E. Erikss.
Neoschizotheciaceae S.K. Huang & K.D. Hyde
89. Cercophora caudata (Sacc.) N. Lundq., recollected in China
Sporidesmiales Crous
Sporidesmiaceae Fr.
90. Sporidesmium tratense J. Yang & K.D. Hyde, sp. nov.
91. Sporidesmium versicolor J. Yang & K.D. Hyde, sp. nov.
Xenospadicoidales Hern.-Restr., J. Mena & Gené
Xenospadicoidaceae Hern.-Restr., J. Mena & Gené
92. Neospadicoides biseptata J. Yang, L.L. Liu & K.D. Hyde, sp. nov.
Xylariales genera incertae sedis
93. Stanjehughesia aquatica J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Sordariomycetes families incertae sedis
Acrodictyaceae J.W. Xia & X.G. Zhang
94. Acrodictys chishuiensis J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
95. Acrodictys effusa L.L. Liu, J. Yang & Z.Y. Liu, sp. nov.
96. Acrodictys pyriformis J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Introduction
Freshwater fungi are a taxonomically and ecologically diverse group distributed worldwide. The definition of freshwater fungi by Thomas (1996) is commonly followed in current studies as “fungi that for the whole or part of their life cycle relies on freshwater, or which uses any resource of a predominantly aquatic or semi-aquatic nature as a substratum”. El-Elimat et al. (2021) added the phylogenetic sense to the definition that “the species belong to exclusive freshwater and/or aquatic phylogenetic lineages as freshwater indweller species and that are phylogenetically related to terrestrial lineages as freshwater immigrant species”. Freshwater fungi occur in various freshwater habitats, such as ponds, lakes, rivers, streams, swamps, water in tree holes and artificial habitats including pools, reservoirs, dams, drainage ditches, and water-cooling towers (Shearer 1993; Thomas 1996; Shearer et al. 2004; Hyde et al. 2016a; Grossart et al. 2019). They comprise a diverse assemblage of saprobes, parasites, pathogens, endophytes, and mutualistic taxa on dead, decaying plant litter or associated with other fungi, aquatic macrophytes, algae, and fish. In freshwater ecosystems, they play an essential role in the decomposition and mineralization of organic matter (Wong et al. 1998b; Wurzbacher et al. 2011, 2014; Jones et al. 2014). Taxonomically, they distributed in 13 fungal phyla: Aphelidiomycota, Ascomycota, Basidiomycota, Blastocladiomycota, Chytridiomycota, Entomophthoromycota, Monoblepharomycota, Mortierellomycota, Mucoromycota, Olpidiomycota, Rozellomycota, Sanchytriomycota and Zoopagomycota. (Calabon et al. 2020b, 2022). The most speciose phylum is Ascomycota, with freshwater fungi most reported in Dothideomycetes and Sordariomycetes and a few in Eurotiomycetes (Jones et al. 2014; Liu et al. 2015b; Luo et al. 2019; Wang et al. 2019; Dong et al. 2020b; Réblová et al. 2020a; Calabon et al. 2022).
Karst landscapes are sculpted largely by the dissolution of soluble rocks such as limestone, dolomite, and gypsum by the action of groundwater or surface water (de Waele et al. 2009). It rarely develops on more weakly soluble rocks such as granite and quartzite (Simms 2014). Karst landscapes are characterized by underground drainage systems, caves, sinkholes, springs, towers, or natural bridges. The South China Karst has been a Natural World Heritage Serial Site since June 2007, spanning the provinces of Chongqing, Guangxi, Guizhou, and Yunnan. Guizhou Province, especially, owns 62% of the land as karst landforms (109,100 km2) with rich types and best development, ranking top in China (Liu et al. 2018). This area is a biodiversity hotspot in China, containing abundant rare, endangered, and indigenous plant and animal species. Hence, it is likely that a large number of fungal species exist in the Guizhou karst region. In Thailand, karst is widespread mainly in the west, covering 18% of the area of about 50,000 km2 and crossing 11 degrees of latitude (19.3° N to 8.5° N) (Zhang et al. 2014a). The typical karst landscapes are well-developed in Thailand, including plateau polje, peak cluster, peak valley, and offshore peak forest with high biodiversity (Zhang et al. 2014a). Studies have been carried out to investigate the diversity, taxonomy, and phylogeny of fungi from rocks and caves from karst regions in Guizhou Province, China, and Thailand, but rarely on freshwater fungi (Reeves 2000; Gorbushina et al. 2003; Pedro and Bononi 2007; Feeser and O’Connell 2010; Zhang et al. 2017a; Chen et al. 2020a, 2021).
In this study, we concentrate on lignicolous freshwater ascomycetes and aim to identify these fungi and resolve their systematic placement. Fresh collections were obtained from decaying submerged wood in lakes and streams, mainly from karst regions in China (Guizhou Province) and Thailand, and some are from the danxia landscape in Guizhou. The taxonomy of more than 90 species is resolved. Based on the morphology and molecular DNA data, four new genera and 47 new species are introduced with descriptions and illustrated accounts. Seven new combinations are introduced, ten new geographical records, and nine habitat records are reported. Reference sequences are first provided to elucidate the phylogenetic placement of several previously described species or genera.
Materials and methods
Collection and examination of specimens
Specimens of submerged decaying twigs were collected from streams or lakes, mainly from karst regions in China (Guizhou Province) and Thailand (Chiang Rai, Phang Nga, Prachuap Khiri Khan, Rayong, and Trat Provinces). Some samples were especially collected from the danxia landscape in Chishui City (Guizhou Province, China). Samples were taken to the laboratory in zip-lock plastic bags and incubated in plastic boxes lined with moistened tissue at room temperature for 1 week. Motic SMZ 168 Series (Motic, Xiamen, China) and Nikon SMZ-171 (Nikon, Tokyo, Japan) dissecting microscopes were used to observe the fungal colonies and fruiting bodies. Fungal structures were examined and photographed using a Nikon ECLIPSE 80i (Nikon, Tokyo, Japan) compound microscope fitted with a Canon 600D/70D (Canon, Tokyo, Japan) digital camera. Single spore isolations were made onto water agar (WA) or potato dextrose agar (PDA), and germinated spores were transferred onto malt extract agar (MEA) or PDA following the method in Luo et al. (2018b). Tarosoft Image Frame Work (Tarosoft, Nontha Buri, Thailand) was used for measurement, and images used for figures were processed with Adobe Photoshop CC 2019 (Adobe Systems, San Jose, CA, USA) software. Herbarium specimens were deposited in the herbarium of Mae Fah Luang University (MFLU), Chiang Rai, Thailand, the herbarium of Cryptogams, Kunming Institute of Botany Academia Sinica (HKAS), Kunming, China, and the herbarium of Guizhou Academy of Agricultural Sciences (GZAAS), Guiyang, China. Axenic cultures were deposited in Mae Fah Luang University Culture Collection (MFLUCC) and Guizhou Culture Collection (GZCC). Facesoffungi and Index Fungorum numbers were registered as outlined in Jayasiri et al. (2015) and Index Fungorum (2022).
DNA extraction, PCR amplification, and sequencing
Germinated spores were grown on MEA/PDA medium at 25 °C for 1 month. Fungal mycelium was scraped using a sterilized scalpel and transferred to a 1.5 mL microcentrifuge tube for genomic DNA extraction. An Ezup Column Fungi Genomic DNA Purification Kit (Sangon Biotech, China) was used to extract DNA following the manufacturer’s instructions. DNA was extracted directly from fruiting bodies following the method in Li et al. (2015) for species which failed to germinate or stop growing after germination. DNA amplification was performed by polymerase chain reaction (PCR). LSU, ITS, SSU, TEF1α, and RPB2 gene regions were amplified using the primer pairs LR0R/LR5 (Vilgalys and Hester 1990; Rehner and Samuels 1994), ITS5 or ITS1/ITS4 (White et al. 1990), NS1/NS4 (White et al. 1990), 983F/2218R (Rehner and Buckley 2005), and fRPB2-5F/fRPB2-7cR (Liu et al. 1999). The amplifications were performed in a 25 μL reaction volume containing 9.5 μL ddH2O, 12.5 μL 2 × Taq PCR Master Mix with blue dye (Sangon Biotech, China), 1 μL of DNA template, and 1 μL of each primer (10 μM). The amplification condition for LSU, ITS, SSU, and TEF1α consisted of initial denaturation at 94 °C for 3 min, followed by 40 cycles of 45 s at 94 °C, 50 s at 56 °C, and 1 min at 72 °C, and a final extension period of 10 min at 72 °C. The amplification condition for the RPB2 gene consisted of initial denaturation at 95 °C for 5 min, followed by 37 cycles of 15 s at 95 °C, 50 s at 56 °C, and 2 min at 72 °C, final extension period of 10 min at 72 °C. Purification and sequencing of PCR products were carried out by Shanghai Sangon Biological Engineering Technology and Services Co., Shanghai, China.
Phylogenetic analyses
Five gene markers LSU, ITS, SSU, TEF1α, and RPB2 were used for the multi-gene analyses with the whole or part of them concatenated for different fungal groups. Single-locus sequences were aligned using the online multiple alignment program MAFFT v.7 (Available online: http://mafft.cbrc.jp/alignment/server/; Katoh and Standley 2013; Kuraku et al. 2013; Katoh et al. 2017). The alignments were checked visually and improved manually where necessary using AliView v. 1.19-beta1k (Larsson 2014) or trimmed using the Multiple Alignment Trimming tool in TBtools (Chen et al. 2020b) with 0.5 site coverage cutoff value. The concatenated sequence alignments were obtained from SequenceMatrix v 1.8 (Vaidya et al. 2011).
Maximum likelihood (ML) and Bayesian inference (BI) analyses were employed to assess phylogenetic relationships. ML and BI analyses were performed through the CIPRES Science Gateway V. 3.3 (Miller et al. 2010). ML analyses were conducted with RAxML-HPC v. 8.2.12 (Stamatakis 2014) using a GTRGAMMA approximation with rapid bootstrap analysis followed by 1000 bootstrap replicates. For the BI approach, MrModeltest2 v. 2.3 (Nylander 2008) was used to infer the appropriate substitution model that would best fit the model of DNA evolution for the combined dataset. BI analyses were performed in a likelihood framework implemented in MrBayes 3.2.6 (Huelsenbeck and Ronquist 2001). Six simultaneous Markov chains were run until the average standard deviation of split frequencies was below 0.01, with trees saved every 1000 generations. The first 25% of saved trees, representing the burn-in phase of the analysis, were discarded. The remaining trees were used for calculating posterior probabilities of recovered branches (Larget and Simon 1999).
The resulting trees were printed with FigTree v. 1.4.4, and the layout was created in Adobe Illustrator 2020 (Adobe Systems, San Jose, CA, USA). Sequences generated in this study were deposited in GenBank and listed in Table 1. Taxa used in the phylogenetic analyses and their GenBank accession numbers are listed in the Supplementary material.
Results
Taxa illustrated below are in alphabetical order. They represent a total of 92 species, 76 genera in 44 families, 26 orders and three classes in Ascomycota (Figs. 1, 46, 50, 53).
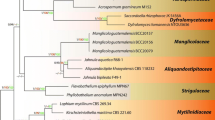
Maximum likelihood majority rule consensus tree for Dothideomycetes isolates using LSU, SSU, TEF1α, and RPB2 sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated above branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Orbilia auricolor (AFTOL-ID 906) and Orbilia vinosa (AFTOL-ID 905). The new collections are in bold with new taxa in blue. Families and orders are indicated with colored blocks. Branches with 100% ML BS and 1.0 PP are thickened
Dothideomycetes O.E. Erikss & Winka
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss.
Notes: Asterinales comprises epifoliar fungi commonly known as black mildews on living leaves (Hosagoudar et al. 2013). The order is characterized by orbicular, dark, flattened thyriothecia with dehiscent, star-like openings, net-like hyphae and appressoria present or absent, globose, ellipsoidal or clavate asci and asymmetrically ellipsoidal, clavate or fusiform, brown, uniseptate ascospores. Illustrations and discussions of the morphological characters and phylogenetic placement of Asterinales taxa are detailed in Hongsanan et al. (2020b).
Stictographaceae D.Q. Dai & K.D. Hyde
Notes: Stictographaceae accommodates five lichenicolous or saprobic genera with cymbiform to lirelliform, or a slit-like disc, black to dark brown ascomata, broadly clavate to subglobose asci and ellipsoid, brown, 1-septate ascospores (Dai et al. 2018; Hongsanan et al. 2020b). The asexual state is still unknown to the family. In this study, based on molecular analysis, we identify a dematiaceous hyphomycete as a member of Actinocladium and place it in Stictographaceae.
Actinocladium Ehrenb.
Notes: Actinocladium was introduced by Ehrenberg (1819) with A. rhodosporum as the type species. The scientific description and drawing of the generic type were provided by Ellis (1971).
Actinocladium aquaticum J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF900126; Facesoffungi number: FoF12776; Fig. 2
Etymology: referring to the aquatic habitat of the fungus.
Holotype: GZAAS 22-2201
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on substrate superficial, effuse, hairy, dark brown, scattered or in small groups. Mycelium mostly immersed, composed of branched, septate, pale brown to brown hyphae. Conidiophores macronematous, mononematous, solitary or in small groups, erect, cylindrical, straight or slightly flexuous, smooth-walled, 1–3-septate, dark brown, slightly paler and narrower towards the apex, 36–64 × 3.5–4.5 µm (\({\overline{\text{x}}}\) = 51 × 4 µm, n = 15). Conidiogenous cells monoblastic, integrated, cylindrical, terminal, determinate, brown, truncate at the apex. Conidia acrogenous, solitary, obpyramidal, with 2–4 arms, septate, brown, 15–20 µm high from the base to the arms intersection from the front view, 13–18 µm wide, arms 6–29 µm long. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h and germ tubes produced from the apex of each arm. Colonies growing on PDA medium reaching 10 mm in 2 weeks at 25 °C in dark, circular, with velvety aerial mycelium, grayish white in the middle and dark brown at the edge; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS27-4 (GZAAS 22-2201, holotype), ex-type culture GZCC 20-0504.
Notes: Actinocladium (Ehrenberg 1819) and Triposporium (Corda 1837) are two old hyphomycetous genera that are poorly studied due to the lack of type specimens or scientific description for some species, e.g., A. minimum, A. penicillus, T. muricatum and T. patavinum. Species in the two genera were transferred from or to other genera, such as Ceratosporella which is clearly distinguished from Triposporium and Actinocladium by the conidial branches (arms) arising from a single basal cell (Wu and Zhuang 2005). Actinocladium and Triposporium species share the morphological characteristics in macronematous, mononematous, brown conidiophores, monoblastic conidiogenous cells with or without percurrent proliferation, and brown, stauriform conidia with obpyramidal lower part and mostly 3–4 arms extending upwards and outwards. Hughes (1951) and Ellis (1971) described the conidial formation mechanism of the type species of Triposporium and Actinocladium, respectively. They share a similar conidial formation except for the number of stalk cells under the initial swelling where the arms diverge. Triposporium elegans has a one-celled stalk, while Actinocladium rhodosporum produces a 1–4-celled stalk. However, it becomes challenging to classify the species when only one-celled stalks are present. Species described in the two genera did not always follow the generic delimitation that makes their taxonomy unclear (Hughes 1951; Ellis 1971; Kuthubutheen and Nawawi 1991; Matsushima 1993; Wu and Zhuang 2005; Manoharachary et al. 2010). Because of the narrow delimitation of Actinocladium and Triposporium, we prefer to consider them congeneric until molecular data become available.
In this study, we collected a freshwater fungus resembling Actinocladium and Triposporium, characterized by non-percurrent conidiogenous cells and stauriform conidia with a short cylindrical basal cell which is considered as the stalk cell (Hughes 1951; Ellis 1971) and arms extending from the penultimate cell. This fungus matches the conidial formation of Triposporium elegans and Actinocladium rhodosporum (when one-celled stalk cells are present). However, the short conidiophores, conidial shape and cells arrangement are comparable to the latter (Hughes 1951; Ellis 1971; Kirschner et al. 2001; Wu and Zhuang 2005). We therefore treat it in Actinocladium as a new species A. aquaticum.
Actinocladium aquaticum is similar to A. rhodosporum, A. agumbense, and Triposporium lambdaseptatum in conidial morphology but distinguished by shorter conidiophores (Actinocladium rhodosporum up to 130 µm long, Actinocladium agumbense 25–75 µm long, Triposporium lambdaseptatum 40–80 µm long), non-percurrent conidiogenous cells and shorter arms (Actinocladium rhodosporum up to 140 µm long, Actinocladium agumbense 35–50 µm long, Triposporium lambdaseptatum 25–70 µm long) (Ellis 1971; Kuthubutheen and Nawawi 1991; Manoharachary et al. 2010).
In the phylogenetic analysis inferred from LSU, SSU, TEF1α, and RPB2 sequence data, A. aquaticum formed a single branch within Stictographaceae but was weakly supported (Fig. 1). Among the taxa in Asterinales, a triposporium-like asexual morph is known for Batistinula gallesiae (Asterinaceae) of which stauriform conidia develop on the external mycelium (Guatimosim et al. 2015). Actinocladium aquaticum likely belongs to the order Asterinales, although with weak support and being the first asexual morph reported in Stictographaceae.
Kirschsteiniotheliales Hern.-Restr., R.F. Castañeda, Gené & Crous
Notes: Kirschsteiniotheliales contains a family Kirschsteiniotheliaceae and three species in Astrosphaeriella, Brachysporiella, and Solicorynespora (Hernández-Restrepo et al. 2017). Phylogenetic and molecular clock analyses confirmed its ordinal ranking, but the systematic placement is unstable in Dothideomycetes (Hernández-Restrepo et al. 2017; Hongsanan et al. 2020b).
Kirschsteiniotheliaceae Boonmee & K.D. Hyde
Notes: Kirschsteiniotheliaceae was introduced by Boonmee et al. (2012) to accommodate the holomorphic genus Kirschsteiniothelia based on the phylogeny inferred from combined LSU, SSU, and ITS gene regions. Taxa in the family are widely distributed and commonly saprobic on bark, dead twigs, branches, and stems from terrestrial and aquatic habitats. Some also occurred on leaves or survived in sparkling wine.
Kirschsteiniothelia D. Hawksw.
Notes: Based on the morphological examinations of type material and additional specimens, Hawksworth (1985) established Kirschsteiniothelia to accommodate six new combinations similar to Microthelia incrustans and K. aethiops. Kirschsteiniothelia aethiops was designated as the generic type with a large synonym. Kirschsteiniothelia is characterized by superficial, erumpent, papillate, dark brown ascomata, 8-spored or rarely 4-spored, pedicellate asci with an ocular apical chamber and ellipsoidal, usually asymmetrical, verruculose or smooth, uniseptate, rarely 2-septate, olivaceous brown to dark brown ascospores with or without a mucilaginous sheath, sometimes with longitudinal or sinuate furrows visible in face view (Hawksworth 1985; Mehrabi et al. 2017; Hyde et al. 2018). The asexual fungus Dendryphiopsis atra was commonly found in intimate juxtaposition to ascomata of K. aethiops on natural substrates, and their connection was proved by Hughes (1978) in a cultural study. Hughes (1978) found the ascomata fragments of Amphisphaeria incrustans (synonym of K. aethiops) sporulated on agar with conidiophores of D. atra and similar cultures were obtained from single spore isolation of D. atra which grew on the natural substrates. The life history of Kirschsteiniothelia with Dendryphiopsis asexual state was further confirmed experimentally with molecular evidence (Boonmee et al. 2012). Recently, sporidesmium-like asexual morphs were frequently reported in Kirschsteiniothelia but without known sexual morphs (Li et al. 2016a; Su et al. 2016a; Hyde et al. 2017b; Bao et al. 2018; Sun et al. 2021).
Kirschsteiniothelia dushanensis L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
Index Fungorum number: IF559444; Facesoffungi number: FoF12777; Fig. 3
Etymology: referring to the collecting location at Dushan District in China.
Holotype: GZAAS 20-0310
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, dark brown, hairy, glistening. Mycelium mostly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight or slightly curved, cylindrical, slightly narrower towards the apex, rounded and darkened at the apex, verrucose, septate, unbranched, dark brown, (120–)160–307 µm long, 6.5–13 μm wide near the base. Conidiogenous cells monoblastic, integrated, terminal, determinate, sometimes elongating percurrently, cylindrical or doliform, brown, darkened at the apex and proliferating portion, 9–26(–45) × 3–7 μm. Conidia acrogenous, solitary, rostrate, 5–11-septate, with distoseptate, fusiform lower part and euseptate, narrower cylindrical upper part, olivaceous brown to soot brown, pale brown or subhyaline at the apex, 62–81 × 12.5–18 μm (\({\overline{\text{x}}}\) = 77 × 16 μm, n = 20), smooth-walled, truncate and darkened at the base, rarely proliferating, sometimes with a mucilaginous sheath surrounding the tail-like upper part or the apex. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium within 24 h and germ tube produced from the apex. Colonies on PDA medium reaching 5–10 mm diam. after 2 weeks at 25 °C in dark, circular, ring-like, greyish green on the surface, with dense, velvety aerial mycelium, in reverse dark green with entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.917° N, 107.617° E, on decaying wood submerged in a freshwater stream, 5 July 2018, L.L. Liu, 18D-43 (GZAAS 20-0310, holotype), ex-type culture GZCC 19-0415.
Notes: Kirschsteiniothelia dushanensis is clearly distinguished from Dendryphiopsis asexual morphs in the genus which have branched conidiophores and ellipsoidal conidia. Sporidesmium-like taxa belonging to Kirschsteiniothelia are K. dushanensis, K. aquatica, K. cangshanensis, K. fluminicola, K. rostrata, K. submersa, K. thailandica and K. tectonae. They can be separated by the conidial shape and dimensions of conidia and conidiophores and are distinct from molecular data. In the phylogenetic analysis, K. dushanensis formed a sister group to K. fluminicola (Fig. 1). They share the morphological traits of macronematous, mononematous conidiophores with rounded apex and obclavate, rostrate conidia with similar length. Conidia of K. dushanensis are olivaceous brown while that are pale brown in K. fluminicola (Bao et al. 2018). Conidial proliferation was observed in both species, but a mucilaginous sheath was only described in K. dushanensis. Comparisons of the LSU and ITS sequence of K. dushanensis (GZCC 19-0415) and K. fluminicola (MFLUCC 16-1263) showed 98.49% (785/797 base pairs (bp), one gap) and 90% (405/450 bp, seven gaps) similarity, respectively. Kirschsteiniothelia dushanensis resembles Sporidesmium ghanaense and S. gyrinomorphum with percurrent conidiogenous cells and similar conidial shape with a fusiform lower part and narrower cylindrical tail-like upper part. However, conidia of S. gyrinomorphum [80–160 × 16.5–22 μm (\({\overline{\text{x}}}\) = 105 × 20 μm)] are reddish brown and larger than the other two while in K. dushanensis [62–81 × 12.5–18 μm (\({\overline{\text{x}}}\) = 77 × 16 μm)] they are olivaceous brown and in S. ghanaense (31–53 × 10–14 µm) dark brown at the broadest part and paler at the base and upper part (Ellis 1958; Yang et al. 2018a). A mucilaginous sheath is present in K. dushanensis but absent in the others. Kirschsteiniothelia dushanensis and S. gyrinomorphum have a rounded apex of the conidiogenous cells while truncated in S. ghanaense. Conidiophores in S. gyrinomorphum (360–740 μm long) are much longer than that in K. dushanensis (120–307 μm long) and S. ghanaense (40–130 μm long) (Ellis 1958; Yang et al. 2018a). Kirschsteiniothelia dushanensis is a member in Dothideomycetes while S. gyrinomorphum belongs to Sordariomycetes. Molecular data is unavailable for S. ghanaense.
Natipusillales Raja, Shearer, A.N. Mill. & K.D. Hyde
Notes: Based on the phylogenetic analysis of combined LSU and SSU sequence data, Hyde et al. (2013) introduced the monotypic order Natipusillales in Dothideomycetes incertae sedis (Hongsanan et al. 2020b).
Natipusillaceae Raja, Shearer & A.N. Mill.
Notes: Natipusillaceae is a monotypic family with Natipusilla as the type genus (Raja et al. 2012).
Natipusilla A. Ferrer, A.N. Mill. & Shearer
Notes: Natipusilla was introduced to accommodate three ascomycetes N. decorospora, N. limonensis and N. naponensis which were collected on submerged, decorticated or corticated woody debris from freshwater streams or swamps in America (Ferrer et al. 2011). Later, Raja et al. (2012) described another species N. bellaspora, also on decorticated wood submerged in a freshwater stream in Peru. Natipusilla is characterized by superficial, small, globose, hyaline to pale brown ascomata, non or few pseudoparaphyses, globose to subglobose or broadly ellipsoidal asci and clavate or asymmetrically fusiform, hyaline, 1-septate to tardily 2- or 3-septate ascospores with or without a gelatinous sheath. The asexual state remains unknown to the genus.
Natipusilla limonensis A. Ferrer, A.N. Mill. & Shearer, Mycologia 103(2): 417 (2011)
Index Fungorum number: IF518367; Facesoffungi number: FoF12778; Fig. 4
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 90–120 μm diam., scattered or gregarious, superficial, perithecial, globose, hyaline becoming light brown with age. Ascomatal wall coriaceous, thick-walled, of textura angularis in surface view. Pseudoparaphyses not observed. Asci 40–50 × 22.5–29 µm, few, globose, ellipsoidal or broadly clavate, 8-spored, without an apical chamber, stalk absent. Ascospores 24–33 × 7–10 µm (\({\overline{\text{x}}}\) = 29 × 9 µm, n = 20), clavate or asymmetrically fusiform, papillate, hyaline, uniseptate, smooth-walled, guttulate, constricted at the septum, upper cell slightly broader than the lower cell, straight or usually slightly curved, surrounded by a mucilaginous shield-shaped sheath.
Culture characteristics: Ascospores germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in natural light, circular, with dense, velvety, olivaceous brown mycelium in the middle and darker mycelium at the edge; in reverse dark brown with entire margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT5-4 (MFLU 22-0064 and HKAS 112149), living cultures MFLUCC 17-2386 and GZCC 20-0375.
Notes: Natipusilla limonensis can be distinguished from other species in the genus by ascospore dimension and the shape of the ascospore sheath. They are well-separated based on molecular DNA data. Our collection matches well with N. limonensis in morphology but has smaller asci and ascospores than the holotype. This may be because the fungus was still young when observed. The phylogenetic analysis revealed our collection clustered together with Natipusilla limonensi (ILL AF286-1 and PE3-2a) with strong support (Fig. 1). Thus, our collection is identified as N. limonensis based on morphology and molecular evidence. Natipusilla limonensis is known in America and Peru from freshwater habitats (Ferrer et al. 2011; Raja et al. 2012). This study extends the known geographical distribution of this species to Thailand.
Pleosporales Luttr. ex M.E. Barr
Notes: In a recent updated review of the order, Pleosporales comprises 91 families, the largest order in Dothideomycetes (Hongsanan et al. 2020a). Taxa in the order can be epiphytes, endophytes, parasites, saprobes, hyperparasites or lichenized in various habitats and have a worldwide distribution. Pleosporales possesses perithecioid, papillate ascomata, bitunicate, generally fissitunicate asci and mostly septate ascospores in different colors and shapes with or without a gelatinous sheath and with coelomycetous asexual morphs more than its hyphomycetes (Zhang et al. 2012a). Besides the morphological characteristics, some biological traits such as metabolite production, substrate staining reactions and host spectrum (Zhang et al. 2009c) are also used to delimit families in the order.
Amniculicolaceae Yin. Zhang, C.L. Schoch, J. Fourn., Crous & K.D. Hyde
Notes: Amniculicolaceae comprises five sexual genera, Amniculicola, Fusiformispora, Murispora, Neomassariosphaeria and Pseudomassariosphaeria (Zhang et al. 2009c; Hongsanan et al. 2020a). They have erumpent, immersed or nearly superficial ascomata with a rough black surface, usually stain the substrate purple, short-pedicellate asci with an ocular chamber and narrowly or broadly fusiform, hyaline to brown, 1 to multi-septate or muriform ascospores, straight or slightly curved, constricted at the septa and often with a gelatinous sheath. Asexual morphs of the family are coelomycetous of Murispora asexualis, M. fissilispora and M. hawksworthii (Wanasinghe et al. 2015; Magaña-Dueñas et al. 2020) and hyphomycetous of Anguillospora longissima, Fouskomenomyces cupreorufescens, F. mimiticus and Vargamyces aquaticus (synonym: Repetophragma ontariense) (Zhang et al. 2009d; Hernández-Restrepo et al. 2017). Members in the family are mainly lignicolous saprobic fungi from freshwater and terrestrial habitats widespread in Europe and known in China and Thailand in Asia (Zhang et al. 2009c; Wanasinghe et al. 2015; Hernández-Restrepo et al. 2017; Hyde et al. 2019; Dong et al. 2020b; Magaña-Dueñas et al. 2020; Phukhamsakda et al. 2020).
Amniculicola Y. Zhang & K.D. Hyde
Notes: Amniculicola contains seven freshwater fungi (Zhang et al. 2008, 2009d; Rossman et al. 2016; Hyde et al. 2019; Magaña-Dueñas et al. 2022). Five sexual morphs have immersed to superficial ascomata with a slit-like ostiole or a papilla, narrowly cylindrical, short-pedicellate asci with a truncate ocular chamber and hyaline, 1–3-septate ascospores sometimes with a gelatinous sheath, often staining the woody substrates purple (Zhang et al. 2008). Phylogenetic analyses supported the placement of a hyphomycetous fungus Anguillospora longissima (synonym: Amniculicola longissima, Rossman et al. 2016) nested in the Amniculicola clade. Amniculicola immersa grouped with Fouskomenomyces (Hongsanan et al. 2020a; this study). Recently, a coelomycetous fungus Amniculicola asexualis was introduced to the genus (Magaña-Dueñas et al. 2022). In this study, we report a sexual morph of A. asexualis (Fig. 5).
Maximum likelihood majority rule consensus tree for Amniculicolaceae using LSU, ITS, SSU, and TEF1α sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated near branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Leptosphaeria dolium (CBS 505.75 and CBS 125979). The new taxa are in blue and new collection in bold. The ex-type strains are indicated with T after the strain number. Branches with 100% ML BS and 1.0 PP are thickened
Amniculicola asexualis Magaña-Dueñas, Stchigel & Cano, J Fungi 8(8, no. 849): 12 (2022)
Index Fungorum number: IF842769; Facesoffungi number: FoF12779; Fig. 6
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Coelomycetous. See Magaña-Dueñas et al. (2022). Sexual morph: Ascomata 280–320 μm high, 260–300 μm diam., scattered or gregarious, semi-immersed to superficial, perithecial, subglobose to conical, with basal wall remaining immersed in host tissue, dark brown, ostiolate, papillate, the underlying wood occasionally stained purple. Ascomatal wall coriaceous, 21–38 μm thick, 2-layered, outer layers consisting of dark brown, heavily pigmented, polygonal, thick-walled cells of textura angularis, inner layers consisting of hyaline, elongated, thin-walled cells of textura angularis. Hamathecium of dense, very long trabeculate, filiform pseudoparaphyses, 1.4–2.1 µm wide. Asci 108–166 × 9–11.6 µm (\({\overline{\text{x}}}\) = 132 × 10.5 µm, n = 30), cylindrical, rounded at the apex, 8-spored, fissitunicate, with a truncate ocular chamber and a short, twisted, furcate pedicel. Ascospores 21.4–27.3 × 4.2–6 µm (\({\overline{\text{x}}}\) = 24 × 5 µm, n = 35), partially overlapping, arranged obliquely uniseriate, narrowly fusiform with narrowly rounded to acute ends, the upper cell usually slightly broader and shorter than the lower cell, hyaline, uniseptate, smooth-walled, guttulate, strongly constricted at the septum, sometimes curved.
Culture characteristics: Ascospores germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in dark, circular, with dense, velvety, dark brown mycelium on the surface; in reverse dark brown with filiform margin.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying wood submerged in Suoluo River, 17 October 2018, J. Yang, GDT34-1 (HKAS 112639), living culture GZCC 20-0482.
Notes: In the phylogenetic tree, our isolate (GZCC 20-0482) was sister to the ex-type strain of Amniculicola asexualis (FMR 17946) with strong support (Fig. 5). The comparison of their LSU, ITS, and TEF1α sequences showed 100% (865/865 bp), 99.71% (340/341 bp) and 99.06% (737/744 bp, one gap) sequence similarity, respectively. Based on the molecular evidence, we recognize they are the same species. Amniculicola asexualis was described as a coelomycetous asexual morph on plant debris from freshwater in Spain (Magaña-Dueñas et al. 2022). In this study, we identify our collection as the sexual morph of A. asexualis phylogenetically and report a new record of the species in China.
Amniculicola species are similar in morphology and dimensions of ascomata, asci and ascospores, but they can be well separated by molecular data. Amniculicola asexualis is similar to A. guttulata in morphology and only differs by the slightly longer asci [108–166 µm long (\({\overline{\text{x}}}\) = 132 µm) vs. 113–127 µm long (\({\overline{\text{x}}}\) = 120 µm)] (Hyde et al. 2019). A gelatinous sheath was described in A. guttulata but not observed in our specimen. The substrate staining reactions are not apparent in A. guttulata and A. asexualis. Comparisons of the LSU, ITS, and TEF1α sequences of our specimen with A. guttulata revealed 99.88% (860/861 bp), 97.99% (439/448 bp) and 97.45% (725/744 bp) similarity, respectively.
Neomassariosphaeria Yin. Zhang, J. Fourn. & K.D. Hyde
Notes: Based on sequence data, Zhang et al. (2009c) introduced Neomassariosphaeria to accommodate two new combinations that were initially identified as Massariosphaeria typhicola and Massariosphaeria grandispora. Both species lack molecular data generated from the type material and no epitype has been designated. Neomassariosphaeria grandispora (synonym: Massariosphaeria grandispora) was later transferred to Pseudomassariosphaeria distinguished from Neomassariosphaeria by clavate asci and hyaline multi-septate ascospores (Ariyawansa et al. 2015a). Magaña-Dueñas et al. (2020) resurrected the name Massariosphaeria grandispora as the fungus was placed in Lophiostomataceae.
Massariosphaeria typhicola was transferred from Leptosphaeria by Leuchtmann (1984), but the identity of other collections of the species is questionable. The collection ZT 9428 (culture CBS 609.86) from Switzerland is one of the materials on which the new combination for Massariosphaeria typhicola is based (Leuchtmann 1984; Dong et al. 2020b). Massariosphaeria typhicola ZT 9428 (culture CBS 609.86) grouped within Lindgomycetaceae and was assigned to a new genus Aquimassariosphaeria by Dong et al. (2020b). The collections KT 667-3 (HHUF 27779, culture MAFF 239218) and KT 797 (HHUF 27785, culture MAFF 239219) were found in Japan on dead stems or twigs of unknown herbaceous or woody plants (Tanaka and Harada 2004). The collections KT 667-3 and KT 797 grouped in a sister clade to Aquimassariosphaeria (Lindgomycetaceae) with weak support (Dong et al. 2020b) and recognized as a Massariosphaeria sp. The specimen IFRD 2018 was collected from Denmark. Its connection with the culture CBS 123126 (LSU: GU301844, SSU: GU296174, RPB2: GU371795) was not mentioned in Zhang et al. (2009c, d, 2012a) but confirmed by the author. Massariosphaeria typhicola IFRD 2018 (culture CBS 123126) clustered within Amniculicolaceae and was sister to M. grandispora. They were accommodated in Neomassariosphaeria (Zhang et al. 2009c). Dong et al. (2020b) reexamined the Danish specimen IFRD 2018 (culture CBS 123126). They suggested it differed from M. typhicola (Leuchtmann 1984) by lenticular ascomata with an elongate papilla on the substrate and dark brown to black, reddish-brown ascospores.
Neomassariosphaeria aquimucosa L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu sp. nov.
Index Fungorum number: IF559446; Facesoffungi number: FoF12780; Fig. 7
Neomassariosphaeria aquimucosa (GZAAS 20-0395, holotype) a Colony on wood. b Immersed ascoma. c Section of an ascoma. d Section of peridium. e Pseudoparaphyses. f–l Asci. m–r Ascospores. s Apex of an ascus. t Germinated ascospore. u, v Culture, u from above, v from below. Scale bars: c, d = 50 μm, e–m, r = 30 μm, s, t = 20 μm, n–q = 15 μm
Etymology: referring to the aquatic habitat of the species and a gelatinous sheath of ascospores.
Holotype: GZAAS 20-0395
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata (148–)225–303 × (116–)164–255 μm, scattered or gregarious, immersed, lenticular or depressed ellipsoidal, papillate, ostiolate, staining the substrate purple, visible as a black, slightly protruding elongate papilla on the substrate. Ascomatal wall coriaceous, 19–65 μm thick, consisting of several layers of polygonal, pale brown to hyaline cells of textura angularis. Hamathecium composed of dense, filiform, septate pseudoparaphyses, 1.5–3 µm wide, anastomosing above the asci. Asci 111–188 × 15–20 μm (\({\overline{\text{x}}}\) = 131.5 × 17.8 μm, n = 20), bitunicate, fissitunicate, cylindrical-clavate, straight or curved, 8-spored, with a subapical ocular chamber and a short furcate pedicel. Ascospores 28–40 × 6–10 μm (\({\overline{\text{x}}}\) = 34.5 × 7.7 μm, n = 30), overlapping, 2–3-seriate, fusiform, slightly curved, pale yellow when young, yellowish brown when mature, often broadest at the third cell from the apex, the apical cell usually broader than the basal cell, rounded at both ends, 5–8-septate, constricted at the septa, verruculose, sometimes with a gelatinous sheath.
Cultural characteristics: Ascospores germinating on WA medium within 24 h and germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in dark, circular, matt, producing reddish pigment, white to pale pink on the surface, aerial mycelium velvety, dense in the middle, sparser towards the edge; in reverse reddish brown with entire margin.
Material examined: CHINA, Guizhou Province, Bijie City, Weining, Caohai national nature reserve, 26.817° N, 104.217° E, on decaying aquatic plants submerged in Caohai Lake, 4 October 2018, L.L. Liu, 18C-22 (GZAAS 20-0395, holotype), ex-type culture GZCC 19-0500; ibid, 18C-31 (GZAAS 20-0396, paratype), ex-paratype culture GZCC 19-0501; ibid, 18C-32 (GZAAS 20-0397, paratype), ex-paratype culture GZCC 19-0502; ibid, 18C-33 (GZAAS 20-0398, paratype), ex-paratype culture GZCC 19-0503.
Notes: Neomassariosphaeria aquimucosa is similar to N. typhicola in the morphology of the ascomata, asci and ascospores with overlapping dimensions (Zhang et al. 2012a; Dong et al. 2020b). Asci of N. aquimucosa are longer than N. typhicola (111–188 μm vs. 90–160 μm) (Zhang et al. 2012a; Dong et al. 2020b). They share verruculose, yellowish-brown (when fresh) ascospores with a gelatinous sheath. However, ascospores of N. aquimucosa are mostly 6–7-septate, fusiform, with the apical cell broader than the basal cell, while in N. typhicola they are often 7-septate, symmetrically narrower at both ends. Phylogenetically, they formed a well-separate sister clade in Amniculicolaceae (Figs. 1, 5).
Aquasubmersaceae A. Hashim. & Kaz. Tanaka
Notes: Based on the phylogeny inferred from SSU-ITS-LSU-TEF1α-RPB2 sequences, Hashimoto et al. (2017) established the family Aquasubmersaceae to accommodate Aquasubmersa. The family is phylogenetically close to Lophiotremataceae and Cryptocoryneaceae (Hongsanan et al. 2020a).
Aquasubmersa K.D. Hyde & H. Zhang
Notes: Aquasubmersa was introduced to accommodate a coelomycetous taxon A. mircensis which was collected on submerged decayed wood from a freshwater habitat in Thailand (Zhang et al. 2012b). Later, A. japonica, the sexual stage, was discovered in terrestrial and freshwater habitats in Japan and produced a coelomycetous asexual morph in culture (Ariyawansa et al. 2015a). Aquasubmersa species are characterized by immersed to semi-immersed ascomata with an ostiolate papilla, trabeculate pseudoparaphyses, short-pedicellate asci with an ocular chamber and fusiform, hyaline, uniseptate ascospores with a gelatinous sheath; dark brown to black, semi-immersed to superficial, ostiolate conidiomata, conidiophores reduced to holoblastic, subcylindrical to lageniform conidiogenous cells with ellipsoidal, hyaline, aseptate conidia.
Aquasubmersa japonica A. Hashim. & Kaz. Tanaka, Fungal Divers 75: 87 (2015)
Index Fungorum number: IF551422; Facesoffungi number: FoF00956; Fig. 8
Aquasubmersa japonica (MFLU 15–1157) a, b Ascomata on wood. c Section of an ascoma. d Section of peridium. e Pseudoparaphyses. f, g–l Asci. m–r Ascospores. s Germinated spore. t, u Culture, t from above, u from below. Scale bars: a = 500 μm, b = 100 μm, c = 50 μm, d, m–r = 10 μm, e, g–l, s = 20 μm, f = 30 μm
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 150–200 μm high, 165–210 μm diam., scattered or gregarious, semi-immersed to superficial, perithecial, subglobose, black, ostiolate, papillate. Ascomatal wall coriaceous, 12–35 μm thick, consisting of multi-layered cells of textura angularis, outer layers with black to dark brown, polygonal, thick-walled cells, inner layers with hyaline, polygonal to elongated, thin-walled cells. Hamathecium composed of sparse pseudoparaphyses, 2.5–4 µm wide, cylindrical, trabeculate, hyaline, septate, branched. Asci 63–120(–155) × 10.5–16 µm (\({\overline{\text{x}}}\)= 92 × 12.8 µm, n = 25), bitunicate, cylindrical to clavate, obtuse at the apex with an ocular chamber, 8-spored, straight or slightly flexuous, with a short pedicel. Ascospores 18–22 × 5–7 µm (\({\overline{\text{x}}}\) = 19.7 × 5.5 µm, n = 30), overlapping, obliquely biseriate, fusiform with obtuse ends, straight, hyaline, with a mostly median septum, smooth-walled, guttulate, deeply constricted at the septum, sometimes with a gelatinous sheath.
Culture characteristics: Ascospores germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 5–10 mm diam. after 2 weeks at 25 °C in natural light, circular, with dense, velvety, grayish brown mycelium on the surface; in reverse dark brown with filiform margin.
Material examined: THAILAND, Prachuap Khiri Khan Province, Hua Hin, near Pala-U Waterfall, on decaying wood submerged in a freshwater stream, 25 December 2014, J. van Strien, site4-34-1 (MFLU 15-1157), living culture MFLUCC 15-0622; THAILAND. Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT11-1 (HKAS 112589), living culture MFLUCC 17-2121.
Notes: Our collections match well with the holotype of A. japonica in morphology (Ariyawansa et al. 2015a), though the dimension of asci is larger and slightly shorter and broader ascospores. Comparisons of the LSU, ITS, SSU, TEF1α, and RPB2 sequences of our collections and the holotype of A. japonica revealed 100%, 100%, 99.57%, 100% and 99.6% similarity, respectively. Therefore, we identify our collections as A. japonica and report a new geographical record of this species in Thailand.
Astrosphaeriellaceae Phookamsak & K.D. Hyde
Notes: Astrosphaeriellaceae comprises saprobic or parasitic fungi having dark, conical or mammiform, carbonaceous ascostromata with a minute ostiole and ruptured host remnants around the base; asexual morphos are coelomycetous or hyphomycetous (Phookamsak et al. 2015b; Hongsanan et al. 2020a). In previous phylogenies, the monophyletic clade of Astrosphaeriellaceae was not well-supported (Jayasiri et al. 2019; Dong et al. 2020b; Hongsanan et al. 2020a). Thus, the placement of some genera, e.g., Acuminatispora, Caryospora and Zopfia are not confirmed within the family.
Caryospora De Not.
Notes: Caryospora (de Notaris 1857) and Acrocordiopsis were accommodated in the family Caryosporaceae by Ariyawansa et al. (2015a) based on a multi-gene phylogeny. In our phylogenetic analysis (Fig. 1), Caryospora was placed within Astrosphaeriellaceae which is consistent with the result in Jayasiri et al. (2019), Dong et al. (2020b) and Hongsanan et al. (2020a). Caryospora shares a similar ascomata structure to Astrosphaeriella but differs by broadly fusiform, thick-walled ascospores.
Caryospora aquatica H. Zhang, K.D. Hyde & Ariyawansa, Fungal Divers 75: 54 (2015)
Index Fungorum number: IF551418; Facesoffungi number: FoF00958; Fig. 9
Caryospora aquatica (HKAS 112608) a, b Ascomata on wood. c Section of an ascoma. d Section of peridium. e Pseudoparaphyses. f–n Asci from young to mature. o–u Ascospores. v Ascospores in Indian ink. w Basal part of an ascus. x Germinated ascospore. y, z Culture, y from above, z from below. Scale bars: c = 100 μm, f–n = 50 μm, o, v = 40 μm, d, p–u, w, x = 30 μm, e = 20 μm
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 300–360 μm high, 400–550 μm diam., scattered, erumpent, semi-immersed to superficial, perithecial, subglobose, dark brown to black, ostiolate, papillate. Ostiole periphysate. Ascomatal wall carbonaceous, 23–70 μm thick, consisting of multi-layered cells of textura angularis with brown to hyaline, thick-walled to thin-walled, polygonal or elongated cells. Hamathecium composed of numerous, 1–2 μm wide, filiform, trabeculate, anastomosing pseudoparaphyses, embedded in a gelatinous matrix. Asci 115–255 × 35–67 µm (\({\overline{\text{x}}}\) = 174 × 47 µm, n = 30), bitunicate, fissitunicate, cylindrical to clavate, pedicellate, 8-spored, with an ocular chamber at the apex. Ascospores 34–48 × 17–25 µm (\({\overline{\text{x}}}\) = 41 × 20.5 µm, n = 45), 2–3-seriate, broadly fusiform, hyaline when young, becoming dark olivaceous brown when mature, uniseptate, constricted at the septum, smooth-walled, guttulate, surrounded by a mucilaginous sheath.
Culture characteristics: Ascospores germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 5–10 mm diam. after 2 weeks at 25 °C in dark, circular, with dense, velvety, olivaceous brown mycelium on the surface with hyaline slime; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS21-1 (HKAS 112608), living culture GZCC 20-0499; ibid, CS60-1 (HKAS 112621), living culture GZCC 20-0521.
Notes: Morphological characters and molecular data (LSU and ITS sequences) both support the identification of this collection as C. aquatica (Fig. 1). The species is known in China and Thailand from freshwater habitats. The RPB2 sequence of C. aquatica and the morphology of a fresh collection is provided here.
Pithomyces Berk. & Broome
Notes: Berkeley and Broome (1873) established Pithomyces with P. flavus as the type species. So far, more than 40 species are accepted in the genus but only a few have molecular data. Pratibha and Prabhugaonkar (2015) epitypified P. flavus and proved its connection with the sexual morph Astrosphaeriella vesuvius experimentally with molecular evidence from the same specimen. The Pithomyces flavus name takes priority over the name of the sexual morph, Astrosphaeriella vesuvius (Pratibha and Prabhugaonkar 2015). Pithomyces species with available sequence data mainly clustered in two families, the Astrosphaeriellaceae group including the type species and the Didymosphaeriaceae group which was assigned to a new genus Pseudopithomyces (Ariyawansa et al. 2015a; Wanasinghe et al. 2018a; Hongsanan et al. 2020a). Pithomyces resembles Astrosphaeriella and allied genera with dark, carbonaceous ascomata, trabeculate pseudoparaphyses, long-cylindrical asci with an ocular chamber and septate, narrowly fusiform ascospores (Phookamsak et al. 2015b; Wanasinghe et al. 2018a). It can be distinguished by the hyphomycetous asexual morphs having punctiform or effused, whitish, yellowish, or brown colonies, hyaline, light yellow or pale brown, smooth- or rough-walled hyphae, conspicuous or reduced conidiophores, mono or polyblastic conidiogenous cells and obovoid, ellipsoidal or broadly fusiform, brown, verruculose to spinulose, septate conidia (Ellis 1960; Ariyawansa et al. 2015a; Wanasinghe et al. 2018a).
Pithomyces flavus Berk. & Broome, J Linn Soc, Bot 14(74): 100 (1873) [1875]
Index Fungorum number: IF224189; Facesoffungi number: FoF11464; Fig. 10
Synonym: Astrosphaeriella vesuvius (Berk. & Broome) D. Hawksw. & Boise, Sydowia 38: 122 (1986) [1985]
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on substrate scattered, velvety, light yellow to orange yellow. Mycelium superficial, composed of pale brown to yellow, verruculose, branched hyphae, 2.8–3.5 µm wide. Conidiophores arising laterally and irregularly on the hyphae, mononematous, macronematous or reduced, verruculose. Conidiogenous cells monoblastic. Conidia acrogenous, solitary, dry, mid brown to dark brown, ellipsoidal to broadly fusiform, verruculose to spinulose, 4–6-euseptate, darkened at the septa, 25–42 × 17–25 µm (\({\overline{\text{x}}}\) = 34 × 20.5 µm, n = 40), slightly constricted at the septa, paler at both end cells. Sexual morph: not observed in this study.
Culture characteristics: Conidia germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on MEA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in natural light, circular, with dense, dark green mycelium in the middle and white mycelium in the outer ring on the surface; in reverse black with entire margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT33-4 (MFLU 22-0074 and HKAS 112163), living cultures MFLUCC 17-2134 and GZCC 20-0408.
Notes: Pithomyces flavus (synonym: Astrosphaeriella vesuvius) was reported on leaf sheaths, petioles, and decaying wood from terrestrial or freshwater habitats in China, India, Sri Lanka, and Thailand (Ellis 1960; Cai et al. 2002a; Pinnoi et al. 2009; Hu et al. 2013; Pratibha and Prabhugaonkar 2015). In this study, the asexual morph of P. flavus is first reported from a freshwater habitat in Thailand.
Dictyosporiaceae Boonmee & K.D. Hyde
Notes: Dictyosporiaceae is a holomorphic family characterized by perithecial, ostiolate ascomata, cylindrical or clavate asci subsessile or short-pedicellate and fusiform, hyaline or brown, uniseptate ascospores slightly constricted at the septum, with or without a mucilaginous sheath; asexual morphs hyphomycetous possessing cheiroid, digitate, palmate or ellipsoidal conidia or coelomycetous with phialidic conidiogenous cells and ellipsoidal or cylindrical, hyaline or brown, mostly aseptate conidia (Boonmee et al. 2016). Besides the 15 genera listed in Hongsanan et al. (2020a), Immotthia and the newly introduced Pseudocyclothyriella were included in the Dictyosporiaceae (Jiang et al. 2021a).
Digitodesmium P.M. Kirk
Notes: Digitodesmium comprises eight species collected on dead wood, bamboo, and soil from terrestrial or freshwater habitats. The genus resembles Dictyocheirospora and Dictyosporium in cheiroid, digitate non-complanate or complanate conidia. However, it can be distinguished by conidial arms that often separate from the apex in Digitodesmium while usually compact or appressed in the latter two genera (Kirk 1981; Cai et al. 2006; Nóbrega et al. 2021). However, this generic delimitation is not always convincing as Digitodesmium species appear to possess both generic characters of conidia in Dictyocheirospora and Dictyosporium (Ho et al. 1999a; Cai et al. 2002c; Hyde et al. 2019; Nóbrega et al. 2021).
Digitodesmium chishuiense J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF559448; Facesoffungi number: FoF12781; Fig. 12
Etymology: referring to the collecting site at Chishui City in Guizhou Province, China.
Holotype: HKAS 124630
Saprobic on decaying wood in freshwater habitats. Asexual morph: Colonies sporodochial, pulvinate, dark brown to black. Mycelium mostly immersed, composed of brown to hyaline, smooth, thin-walled, septate, branched hyphae. Conidiophores macronematous, mononematous, hyaline to pale brown, smooth, thin-walled, unbranched, cylindrical, 3–4 µm, up to 25 µm long. Conidiogenous cells monoblastic, integrated, terminal, determinate, pale brown, or hyaline, smooth, thin-walled. Conidia acrogenous, holoblastic, solitary, cheiroid, mostly consisting of three arms and rarely 4–5 arms arranged discretely, pale brown or pale reddish brown, multi-septate, (25–)27–32(–35) × 16–21(–24) µm (\({\overline{\text{x}}}\) = 29.5 × 19 µm, n = 60), complanate, with arms closely appressed next to each other and sometimes divergent. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium within 24 h and germ tubes produced from the basal cell. Colonies on PDA medium reaching 5–10 mm diam. in 2 weeks, at 25 °C in dark, circular, with fluffy, dense, velvety, white mycelium on the surface with undulate margin; in reverse yellowish brown in the middle and white at the margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on a decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS39-1 (HKAS 124630, holotype), ex-type culture GZCC 20-0510.
Notes: Digitodesmium possesses semi-macronematous conidiophores, monoblastic conidiogenous cells, cheiroid, digitate, euseptate, complanate or non-complanate conidia, sometimes with appendages or mucilaginous sheath at the apex, septal pore present or absent, arms arising from the basal cell or discrete, often divergent from the apex. The related genera Dictyocheirospora and Dictyosporium have micronematous to macronematous, branched or unbranched conidiophores and similar cheiroid, narrowly balloon-like, non-complanate conidia in the former with arms closely gathered at the apex sometimes divergent, and cheiroid, palmate, complanate conidia in the latter with arms appressed next to each other (Yang et al. 2018b). Among Digitodesmium species, D. chiangmaiense (Hyde et al. 2019), D. heptasporum (Cai et al. 2003b), D. polybrachiatum (Nóbrega et al. 2021) and D. recurvum (Ho et al. 1999a) have dictyocheirospora-like conidia while D. bambusicola (Cai et al. 2002c) produces dictyosporium-like conidia but divergent arms are also present. Molecular DNA data are only available for three species characterized by dictyocheirospora-like or dictyosporium-like conidia which indicated Digitodesmium was the sister clade to Dictyocheirospora (Boonmee et al. 2016; Yang et al. 2018b; Hyde et al. 2019; Nóbrega et al. 2021). It seems that this Digitodesmium clade should be synonymized with Dictyocheirospora. Given the lack of sequence data of the type species and another two with only divergent conidial arms, we avoid proposing nomenclatural changes until the generic criteria are re-evaluated and additional collections or further molecular evidence are provided.
In this study, we collected a dictyosporium-like taxon, Digitodesmium chishuiense, on decaying wood submerged in a freshwater stream. The phylogenetic analysis inferred from ITS-LSU-SSU-TEF1α sequences revealed its placement within Digitodesmium (Fig. 11). Digitodesmium chishuiense is similar to D. bambusicola in sporodochial, pulvinate colonies, unbranched conidiophores and cheiroid, pale brown conidia mostly consisting of three arms united by the basal cell or discrete, complanate when arms appressed and non-complanate when arms divergent. The conidial dimension of D. chishuiense is similar to D. bambusicola (25.5–34.5 × 16.5–24 µm vs. 24–32.5 × 12.5–23 µm) (Cai et al. 2002c). However, the 4–5 conidial arms presented in D. chishuiense are not observed in D. bambusicola. Digitodesmium chishuiense lacks conidial appendages while D. bambusicola has hyaline, subglobose, apical, or subapical appendages on the arms. Molecular data is only available for the ITS and LSU gene regions of a non-type strain of D. bambusicola. With a short overlapping length of the sequences, the ITS and LSU sequences of D. chishuiense differs from D. bambusicola by two bp (310/312 bp) and five bp (546/551 bp), respectively. We recognize D. chishuiense and D. bambusicola as distinct species based on morphological differences. However, further molecular evidence is needed to confirm their relationship.
Maximum likelihood majority rule consensus tree for Dictyosporiaceae using ITS, LSU, SSU, and TEF1α sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated near branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Periconia igniaria (CBS 379.86 and CBS 845.96). The new taxa are in blue. Genera and families are indicated with colored blocks. The ex-type and paratype strains are marked as T and PT, respectively, after the strain number. Branches with 100% ML BS and 1.0 PP are thickened
Didymosphaeriaceae Munk
Notes: Didymosphaeriaceae comprises saprobic, endophytic and pathogenic fungi on leaves, stems or branches from various hosts in terrestrial and aquatic habitats and occasionally as a human pathogen (Munk 1953). The systematic placement of this family was uncertain (von Arx and Müller 1975; Barr 1990; Lumbsch and Huhndorf 2007; Zhang et al. 2012a) until Ariyawansa et al. (2014) synonymized Montagnulaceae under Didymosphaeriaceae within Pleosporales based on a well-resolved phylogeny and morphological comparisons. Currently, 32 genera are accepted in the family that was briefly illustrated in Hongsanan et al. (2020a).
Austropleospora R.G. Shivas & L. Morin
Notes: Morin et al. (2010) introduced Austropleospora to accommodate a plant pathogen A. osteospermi as a sexual morph clustered with the coelomycetous Hendersonia osteospermi, on the host surface. They produced the same asexual morph in culture. The second species, A. archidendri (basionym: Paraconiothyrium archidendri) is invalid because the identifier was not cited in the protologue (Ariyawansa et al. 2015a). Later, Jayasiri et al. (2019) described the coelomycetous A. keteleeriae from the decaying cone of Keteleeria fortunei (Pinaceae) without a known sexual morph. Austropleospora ochracea in sexual morph was newly introduced to the genus by Dissanayake et al. (2021).
Austropleospora ochracea L.S. Dissan, J.C. Kang & K.D. Hyde, Phytotaxa 491(3): 223 (2021)
Index Fungorum number: IF557841; Facesoffungi number: FoF07933, Fig. 13
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata (122–)165–180(–220) μm high, 240–320 μm wide, immersed to semi-immersed, globose to subglobose, scattered, dark brown, papillate, ostiolate. Ascomatal wall coriaceous, 23–35 μm thick, consisting of several layers of polygonal, thick-walled cells of textura angularis, outer layer reddish brown, inner layers yellowish brown becoming paler towards inside. Hamathecium composed of 1–2.5 µm wide, cylindrical, hyaline, septate, branched pseudoparaphyses. Asci 70–107 × 11–16(–19) μm (\({\overline{\text{x}}}\) = 91 × 13.8 μm, n = 20), bitunicate, fissitunicate, clavate, 8-spored, with a small apical chamber and a short furcate pedicel. Ascospores 14–18(–22) × 6.5–10 µm (\({\overline{\text{x}}}\) = 16.5 × 8 µm, n = 20), mostly overlapping uniseriate, ellipsoidal or broadly fusiform with obtuse ends, hyaline to pale yellow when young, becoming yellowish brown to dark olivaceous brown when mature, muriform, smooth-walled, slightly constricted at the middle transvers septum, sometimes with oblique septa.
Cultural characteristics: Ascospores germinating on WA medium and germ tubes produced from one end within 12 h. Colonies on PDA medium reaching 20–25 μm diam. after 20 days at 25 °C in dark, circular, as matted felt with velutinous appearance, white on the surface becoming yellow with age; in reverse white to yellow with entire margin.
Material examined: CHINA, Guizhou Province, Guiyang City, Baihua Lake, 26.655° N, 106.537° E, on decaying branch submerged in a freshwater lake, 18 April 2018, L.L. Liu, 18B-1 (GZAAS 20-0325), living culture GZCC 19-0430.
Notes: Our collection matches the type material of Austropleospora ochracea in the morphology of ascomata, asci and ascospores. However, our collection has slightly larger ascospores [14–18(–22) × 6.5–10 µm (\({\overline{\text{x}}}\) = 16.5 × 8 µm) vs. 10–20 × 5–7 µm (\({\overline{\text{x}}}\) = 15 × 6 µm)] than the holotype (Dissanayake et al. 2021). Mature ascospores of A. ochracea become olivaceous brown which was not observed in the holotype. In the phylogenetic analysis, our collection clustered with A. ochracea (KUMCC 20-0020) as a sister taxon with good statistical support (Fig. 1). Comparison of the LSU, ITS, SSU, and TEF1α sequences of our collection and the holotype of A. ochracea showed 100% (871/871 bp), 99.62% (525/527 bp), 99.88% (850/851 bp) and 100% (920/920 bp) sequence similarity, respectively. We therefore recognize our collection as A. ochracea based on the molecular evidence and extend the known habitat of this fungus to freshwater.
Neokalmusia Ariyaw. & K.D. Hyde
Notes: Neokalmusia was established to accommodate N. brevispora and N. scabrispora that were transferred from Kalmusia (Tanaka et al. 2005; Zhang et al. 2009c; Ariyawansa et al. 2014). Six species are currently accepted in the genus. They were isolated on bamboo culms from terrestrial habitats and known in China, Italy, Japan, and Thailand (Ariyawansa et al. 2014; Dai et al. 2016; Thambugala et al. 2017; Hyde et al. 2020b). Neokalmusia is characterized by immersed or semi-immersed ascomata under the clypeus-like structure composed of host epidermis, pedicellate asci with apical chamber and fusiform, straight, or slightly curved, brown, septate, verrucose or smooth-walled ascospores with or without a mucilaginous sheath. Asexual morph is unknown to the genus.
Neokalmusia aquibrunnea J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559449; Facesoffungi number: FoF12782; Fig. 14
Neokalmusia aquibrunnea (HKAS 112630, holotype) a, b Colony on wood. c Section of an ascoma. d, e Section of peridium. f–k Asci. l, m Pseudoparaphyses. n–t Ascospores. u Germinated ascospore. v, w Colony on PDA medium, v from above, w from below. Scale bars: c = 100 µm, d = 50 µm, e = 30 µm, f–n, u = 20 µm, o–t = 10 µm
Etymology: referring to the aquatic habitat of the species and brown ascospores.
Holotype: HKAS 112630
Saprobic on decaying submerged bamboo culms in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 190–290 μm high, 350–450 μm diam., scattered or gregarious, erumpent, semi-immersed, perithecial, conical or subglobose, dark brown, ostiolate, clypeus-like at surface view, with a small, papillate, pore-like opening at the center. Ascomatal wall coriaceous, 27–50 μm thick, composed of host and fungal tissues, consisting of several layers of dark brown, polygonal, thick-walled cells of textura angularis, uneven thickness, thickened at the side of the base. Hamathecium composed of 1.6–4.3 µm wide, cylindrical, hyaline, septate, branched pseudoparaphyses. Asci 70–129 × 7–10.5 µm (\({\overline{\text{x}}}\) = 91.7 × 8.5 µm, n = 30), bitunicate, fissitunicate, clavate, 8-spored, with a small apical chamber and a long furcate pedicel. Ascospores 10–14 × 3–5 µm (\({\overline{\text{x}}}\) = 12.2 × 3.8 µm, n = 40), biseriate, narrowly fusiform with obtuse ends, gold brown to mid brown, uniseptate, smooth-walled, guttulate, constricted at septum, sometimes slightly curved, with broader upper cell and elongated lower cell.
Culture characteristics: Ascospores germinating on PDA medium within 24 h and germ tubes produced from one end. Colonies growing on PDA medium reaching 10–15 mm in a week at 25 °C in dark, circular, with dense, velvety, grayish brown mycelium on the surface; in reverse dark brown in the middle, paler in the inner ring, dark brown in the outer ring with smooth margin.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying bamboo culms submerged in Suoluo River, 19 October 2016, J. Yang, GD13-1 (HKAS 112630, holotype), ex-type cultures MFLUCC 17-0229 and GZCC 17-0045.
Notes: Among Neokalmusia species, N. aquibrunnea resembles N. arundinis and N. didymospora in having immersed or semi-immersed ascomata, clypeus-like in surface view, clavate, long-pedicellate asci with an apical chamber and asymmetrically fusiform, slightly curved, brown, uniseptate ascospores constricted at the septum, without a mucilaginous sheath (Dai et al. 2016; Thambugala et al. 2017). Neokalmusia didymospora can be distinguished by golden brown, verrucose ascospores and orange to brown clypeus on host surface. Neokalmusia aquibrunnea and N. arundinis share a dark brown clypeus and smooth-walled ascospores of similar dimensions. However, they are phylogenetically distinct (Fig. 1). Comparisons of the ITS, LSU, SSU, and TEF1α sequences of our specimen and the type material of N. arundinis revealed 95.2%, 99.11%, 99.71% and 97.06% sequence similarity, respectively. We follow the guidelines of Jeewon and Hyde (2016) and introduce N. aquibrunnea as a new species and extend the habitat of the genus to freshwater.
Lentitheciaceae Yin. Zhang, C.L. Schoch, J. Fourn., Crous & K.D. Hyde
Notes: Based on a well-supported phylogeny, Zhang et al. (2009c) established the family Lentitheciaceae comprising Lentithecium and several taxa of Katumotoa, Keissleriella, Ophiosphaerella and Stagonospora. Subsequently, several new genera were introduced, and the concept of the family got extended (Hirayama et al. 2010; Quaedvlieg et al. 2013; Wanasinghe et al. 2014; Phookamsak et al. 2015a; Knapp et al. 2015; Tanaka et al. 2015; Wijayawardene et al. 2015; Li et al. 2016a; Dayarathne et al. 2018; Hyde et al. 2020b; Calabon et al. 2021). The family possesses immersed to superficial, globose to lenticular ascomata with papillate, clypeus-like opening or with neck and varied ascospores (fusiform, filiform or ellipsoidal, hyaline, brown or versicolored, 1–3-septate or muriform with or without a mucilaginous sheath); asexual morphs coelomycetous, stagonospora-like or dendrophoma-like (Hongsanan et al. 2020a). Members in the Lentitheciaceae are mostly saprobes thriving on stems, twigs or plant debris of decaying wood or herbaceous plants in terrestrial and aquatic habitats, and rarely as endophytic fungi (Darksidea).
Halobyssothecium Dayarathne, E.B.G. Jones & K.D. Hyde
Notes: Halobyssothecium was established to accommodate a widespread species H. obiones, which mainly grows on salt marsh halophytes and has been assigned to several genera (Dayarathne et al. 2018). Molecular DNA data indicated its close relationship to Lentithecium (Dayarathne et al. 2018; Devadatha et al. 2020; Dong et al. 2020b; Hongsanan et al. 2020a; Calabon et al. 2021). With more fresh collections, Calabon et al. (2021) revised the taxonomy and phylogenetic assessment of Halobyssothecium and Lentithecium. This resulted in an expansion of Halobyssothecium with newly described ascomycetes and coelomycetes and five new combinations transferred from the Lentithecium. Halobyssothecium therefore possesses immersed, semi-immersed, erumpent or superficial, carbonaceous or coriaceous, papillate, ostiolate ascomata, short-pedicellate asci with or without an ocular chamber and asymmetrically or symmetrically fusiform, straight or slightly curved, yellowish-brown or versicolored, 1- or 3-septate, verrucose or smooth-walled ascospores, asexual morphs coelomycetous or hyphomycetous. The remaining species in Lentithecium are sexual stages without known asexual morphs and are distinguished from Halobyssothecium species by hyaline ascospores with a mucilaginous sheath (Zhang et al. 2009a; Tanaka et al. 2015). In our phylogenetic tree, Halobyssothecium formed a sister clade to Lentithecium except for two uncertain species H. versicolor and L. aquaticum (Fig. 15).
Halobyssothecium aquifusiforme J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559450; Facesoffungi number: FoF12783; Fig. 16
Etymology: referring to the aquatic habitat of the species and fusiform ascospores.
Holotype: HKAS 112638
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 220–350 μm high, 160–350 μm diam., scattered or gregarious, immersed, perithecial, subglobose, dark brown, ostiolate, with masses of ascospores at the apex of the short neck. Ascomatal wall coriaceous, 8–26 μm thick, consisting of multi-layered cells of textura angularis, outer layers with brown, polygonal, thick-walled cells, inner layers with pale brown to hyaline, polygonal to elongated, thin-walled cells. Hamathecium composed of 1.2–2.2 µm wide, cylindrical, hyaline, septate, branched pseudoparaphyses. Asci 110–158 × 15–21 µm (\({\overline{\text{x}}}\) = 134 × 17.6 µm, n = 20), bitunicate, cylindrical, rounded at the apex with an ocular chamber, 8-spored, straight, or slightly flexuous, with a short pedicel. Ascospores 26–33 × 8–10 µm (\({\overline{\text{x}}}\) = 29.8 × 8.7 µm, n = 30), overlapping, obliquely biseriate, narrowly fusiform with obtuse ends, yellowish brown, 3-septate, smooth-walled, guttulate, deeply constricted at the septum.
Culture characteristics: Ascospores germinating on PDA medium within 24 h and germ tubes produced from both ends. Colonies growing on PDA medium reaching 5–10 mm in 2 weeks at 25 °C in dark, circular, raised, producing mucilaginous drops in the middle, aerial mycelium dense, velvety, gray in the middle, olivaceous brown at the edge, with white spot at edge of the inner ring; in reverse white in the middle, dark olivaceous at the entire margin.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying wood submerged in Suoluo River, 17 October 2018, J. Yang, GDT29-1 (HKAS 112638, holotype; HKAS 125933, isotype), ex-type cultures MFLUCC 19-0302 and GZCC 20-0481; ibid, GDT45-1 (HKAS 112641, paratype), ex-paratype culture MFLUCC 19-0305.
Notes: In the LSU-ITS-SSU-TEF1α phylogenetic tree, Halobyssothecium aquifusiforme formed a basal branch of the Halobyssothecium clade (Fig. 15). Halobyssothecium aquifusiforme is similar to H. estuariae (Devadatha et al. 2020), H. obiones (Dayarathne et al. 2018) and H. versicolor (Calabon et al. 2021) in the morphology of ascomata, asci and 3-septate, fusiform, straight or slightly curved ascospores slightly constricted at the septa. The former three species share immersed to semi-immersed, uniloculate ascomata while H. versicolor has semi-immersed to superficial, uni- to bi-loculate ascomata. Ascospores of H. estuariae, H. obiones and H. versicolor consist of brown middle cells and hyaline or pale brown end cells, while H. aquifusiforme produces pale yellow to yellowish-brown ascospores (Fig. 16).
Maximum likelihood majority rule consensus tree for Lentitheciaceae using LSU, ITS, SSU, and TEF1α sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated near branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Massarina eburnea (CBS 473.64) and Helminthosporium velutinum (MAFF 243854). The new collections are indicated in bold and new taxa in blue. Ex-type, ex-epitype, ex-lectotype and ex-paratype strains are indicated with T, ET, LT, and PT after the strain numbers. Genera and families are indicated with colored blocks. Branches with 100% ML BS and 1.0 PP are thickened
Halobyssothecium aquifusiforme (HKAS 112638, holotype) a Colony on wood. b Vertical section of ascomata. c Section of an ascoma. d, e Section of peridium. f Paraphyses. g–l Asci. m–s Ascospores. t Germinated ascospore. u, v Colony on PDA medium, u from above, v from below. Scale bars: a = 1000 μm, b = 200 μm, c = 100 μm, d, f–l, t = 30 μm, e, s = 20 μm, m–r = 15 μm
Halobyssothecium caohaiense L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
Index Fungorum number: IF559451; Facesoffungi number: FoF12784; Fig. 17
Halobyssothecium caohaiense (GZAAS 20-0377, holotype) a Colony on natural substrate. b, c Section of a conidioma. d, f–h Conidiogenous cells with conidia. e Section of conidiomatal wall. i Conidia. j Germinated conidium. k, l Culture, k from above, l from below. Scale bars: b, c = 30 μm. e, j = 20 μm. d, f–i = 10 μm
Etymology: referring to the collecting site at Caohai Lake.
Holotype: GZAAS 20-0377
Saprobic on decaying submerged wood in freshwater habitats. Sexual morph: Undetermined. Asexual morph: Conidiomata 174–197 × 206–240 μm, pycnidial, solitary or aggregated, globose to subglobose, glabrous, semi-immersed to superficial, papillate, ostiolate, dark brown to black. Conidiomatal wall 17–36 μm thick, composed of thick-walled, dark brown cells of textura angularis, becoming pale brown to hyaline cells towards inner layers. Conidiophores reduced to conidiogenous cells. Conidiogenous cells monophialidic, hyaline, smooth-walled, cylindrical to lageniform, 5–11 × 1–5 μm (\({\overline{\text{x}}}\) = 7 × 2.5 μm, n = 20). Conidia acrogenous, hyaline, ovoid, ellipsoidal or irregular, thin-walled, smooth-walled, aseptate, 7–12 × 3.5–5.5 μm (\({\overline{\text{x}}}\) = 9.4 × 4.4 μm, n = 40), guttulate.
Culture characteristics: Conidia germinating on WA medium and germ tubes produced from one end within 12 h. Colonies on PDA medium reaching about 35 mm diam. after 2 weeks at 25 °C in dark, circular, consisting of a matted felt with velutinous appearance, grayish green, umbonate, dense in the middle and sparse at the edge; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Bijie City, Weining, Caohai national nature reserve, 26.817° N, 104.217° E, on submerged decaying aquatic plants in Caohai Lake, L.L. Liu, 4 October 2018, 18C-41 (GZAAS 20-0377, holotype), ex-type culture GZCC 19-0482.
Notes: Halobyssothecium estuariae is the only holomorphic species in the genus. Its sexual-asexual link was established by molecular DNA data and fungal association on the same host tissue. Although the two morphs share similar traits of colonies in culture, a connection in vitro is still lacking (Devadatha et al. 2020). Halobyssothecium estuariae is distinguished from our collection by hyphomycetous, xylomyces-like asexual morph. The remaining asexual morphs in the genus are H. kunmingense (Dong et al. 2020b), H. unicellulare (Hyde et al. 2016b), H. bambusicola and H. phragmitis (Calabon et al. 2021), they are similar to our collection in possessing dark brown, immersed to erumpent, uniloculate pycnidia, and hyaline, aseptate, guttulate conidia. They can be separated by conidial shape and dimensions.
In the molecular analysis, our collection grouped with H. unicellulare (MD 6004 and MD 129) and H. carbonneanum (CBS 144076) (Fig. 15). Comparisons of the LSU and ITS sequences of our collection and H. carbonneanum (CBS 144076) revealed 99.7% (709/711 bp) and 99.4% (499/502 bp) similarity, respectively. The LSU sequence showed 99.77% (850/852 bp) similarity between our collection and H. unicellulare. The remaining gene regions are unavailable for H. carbonneanum and H. unicellulare. Halobyssothecium carbonneanum lacks a known asexual morph (Crous et al. 2018b). Halobyssothecium unicellulare and our collection are coelomycetous with mono- to polyblastic, globose to subglobose conidiogenous cells in the former and monophialidic, cylindrical to lageniform conidiogenous cells in the latter. We recognize our collection as a distinct species based on the morphology, but further molecular data are required to confirm their relationship.
Lentithecium K.D. Hyde, J. Fourn. & Yin. Zhang
Notes: Lentithecium was introduced by Zhang et al. (2009a) with L. fluviatile as the type species. Novel taxa and species reassignment were introduced to the genus in several studies (Zhang et al. 2009a; Tanaka et al. 2015; Hyde et al. 2016b; Su et al. 2016b; Crous et al. 2018b; Dong et al. 2020b). Calabon et al. (2021) redefined Lentithecium and accepted four ascomycetes, L. clioninum, L. fluviatile, L. pseudoclioninum and L. aquaticum in the genus. However, L. aquaticum was phylogenetically distinct from the former species (Tanaka et al. 2015; Crous et al. 2018b; Dayarathne et al. 2018; Devadatha et al. 2020; Calabon et al. 2021; this study). The taxonomic placement of L. aquaticum needs to be resolved.
Lentithecium pseudoclioninum Kaz. Tanaka & K. Hiray., Stud Mycol 82: 99 (2015)
Index Fungorum number: IF811309; Facesoffungi number: FoF12785; Fig. 18
Saprobic on decaying submerged twigs in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 164–361.5 × 246.5–460.5 μm, scattered, immersed to partially erumpent, black, globose or subglobose, glabrous, with papilla visible as raised, dark spots on host surface, ostiole central. Peridium 13–33(–86) μm thick, composing several layers of polygonal to subglobose cells of textura angularis, pigmented at the outer layers. Hamathecium comprising numerous, 1–2.5 μm wide, hyaline, septate pseudoparaphyses, embedded in a mucilaginous matrix. Asci 99–130 × 16–22 μm (\({\overline{\text{x}}}\) = 113.5 × 18.5 μm, n = 20), cylindrical-clavate, pedicellate, apically rounded, with an ocular chamber, bitunicate, 8-spored. Ascospores 26–34 × 7–11 μm (\({\overline{\text{x}}} \) = 28.8 × 8.5 μm, n = 30), 2–3-seriate, hyaline, fusiform, uniseptate, constricted at the septum, thin-walled, smooth, slightly curved, guttulate, with a mucilaginous sheath.
Cultural characteristics: Ascospores germinating on WA medium within 24 h and germ tube produced from both ends. Colonies on PDA medium permeated after one mouth at 25 °C in dark, circular, with dense, velvety, brown mycelium on the surface; in reverse, dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Bijie City, Weining, Caohai national nature reserve, 26.817° N, 104.217° E, on submerged decaying aquatic plants in Caohai Lake, L.L. Liu, 12 April 2019, 19C-33 (GZAAS 20-0378), living culture GZCC 19-0483.
Notes: Our specimen matches with L. pseudoclioninum based on the morphology of the holotype given by Tanaka et al. (2015). However, the asci of our specimen are slightly longer [99–130 μm (\({\overline{\text{x}}} \) = 113.5 μm) vs. 62.5–116 μm (\({\overline{\text{x}}} \) = 92 μm)]. Based on further molecular evidence (Fig. 15), we identify our specimen as L. pseudoclioninum and extend its geographical distribution to China.
Setoseptoria Quaedvlieg, Verkley & Crous
Notes: Setoseptoria comprises seven saprobic species commonly collected on Phragmites australis, Scirpus and submerged herbaceous plants (Quaedvlieg et al. 2013). Among them, the type species S. phragmitis is only known as a stagonospora-like coelomycetous stage while the others are ascomycetes with similar morphology. The sexual-asexual connections of Setoseptoria species are not yet confirmed through cultural studies although the link between Stagonospora species and Massarina arundinacea has been suggested several times based on their close association on natural substrates (Tanaka et al. 2015).
Setoseptoria bambusae J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559452; Facesoffungi number: FoF12786; Fig. 19
Etymology: referring to the bamboo host
Holotype: HKAS 112629
Saprobic on decaying submerged bamboo culms in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 120–155 μm high, 280–330 μm diam., scattered or gregarious, immersed under raised host tissue, perithecial, uniloculate, subglobose to conical, dark brown, ostiolate, surface view clypeus-like, with a small pore-like opening at the centre. Ostiole papillate, filled with pale brown to brown cells. Ascomatal wall coriaceous, 13–42 μm thick, consisting of multi-layered cells of textura angularis, with dark brown, larger polygonal, thick-walled cells of outer layers, cells becoming paler, smaller or elongated in inner layers. Hamathecium composed of abundant pseudoparaphyses, 2–3.8 µm wide, cylindrical, hyaline, septate, branched. Asci 130–180 × 14–16 µm (\({\overline{\text{x}}}\) = 155 × 15 µm, n = 20), bitunicate, fissitunicate, cylindrical to clavate, 8-spored, pedicellate, apex rounded with a minute ocular chamber. Ascospores 28–37 × 5.5–7 µm (\({\overline{\text{x}}}\) = 33.2 × 6.2 µm, n = 30), overlapping, biseriate, narrowly fusiform, hyaline, with a nearly median primary septum, 3-septate when mature, smooth-walled, guttulate, deeply constricted at the middle septum, straight or sometimes slightly curved, asymmetrical, often constricted at the upper middle part of the apical cell, surrounded by a large mucilaginous extended sheath.
Culture characteristics: Ascospores germinating on PDA medium within 24 h and germ tubes produced from both ends. Colonies growing on PDA medium reaching 10–15 mm in 2 weeks at 25 °C in dark, circular, with dense, velvety, white mycelium in the middle, grayish brown in the inner ring and daker at the edge; in reverse dark brown with smooth margin.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying bamboo culms submerged in Suoluo River, 19 October 2016, J. Yang, GD12-7 (HKAS 112629, holotype; HKAS 125932, isotype), ex-type cultures MFLUCC 17-0228 and GZCC 17-0044.
Notes: In the phylogenetic analysis inferred from LSU-ITS-SSU-TEF1α sequences (Fig. 15), four strains of S. arundinaceae nested within the genus, but the strains KT552 and KT600 grouped apart from CBS 123131 and CBS 619.86. Since none are type strains with extensive synonyms associated with S. arundinacea, the strains may consist of several morphological assemblages (Tanaka et al. 2015). Setoseptoria arundinaceae (KT552 and KT600) was sister to S. arundelensis with weak bootstrap support (Wanasinghe et al. 2018b; Dong et al. 2020b; this study). They share similar asci and ascospores but differ as the ascomata are immersed, multi-loculate in the former and semi-immersed to superficial, wholly or partly erumpent, uniloculate in the latter (Tanaka et al. 2015; Wanasinghe et al. 2018b). However, comparisons of the LSU sequence of S. arundelensis and S. arundinaceae (KT552 and KT600) revealed 99.88% (854/855 bp) to 100% similarity and ITS sequences showed 99.31% (579/583 bp) similarity. Considering the guidelines of Jeewon and Hyde (2016), S. arundelensis and S. arundinaceae (KT552 and KT600) may be the same species. Re-evaluation will be needed to resolve their classification.
Setoseptoria bambusae clustered with the strain IFRD500-013 which was previously identified as S. arundinaceae but without a description. The LSU and SSU sequences of Setoseptoria bambusae are identical to that of IFRD500-013 and the ITS sequence is different in one nucleotide (551/552 bp). TEF1α sequence is unavailable for the latter. Based on the molecular DNA data, these two strains represent the same taxa. Setoseptoria bambusae is introduced to accommodate our collection and IFRD500-013. Setoseptoria bambusae is indistinguishable from other sexual morphs in Setoseptoria in the morphology of asci and ascospores. However, the asci of Setoseptoria bambusae (130–180 μm long) are shorter than that in S. magniarundinacea (119–200 μm long) but longer than other Setoseptoria species (up to 120 μm) (Tanaka et al. 2015; Hyde et al. 2017b; Wanasinghe et al. 2018b). The ascospores of Setoseptoria bambusae (28–37 × 5.5–7 µm) are smaller than that in S. magniarundinacea (67–82 × 6.5–9 μm) but with similar dimensions to the others.
Towyspora Wanasinghe, E.B.G. Jones & K.D. Hyde
Notes: Towyspora is a monotypic genus typified by T. aestuari (Li et al. 2016a). The genus is characterized by immersed to semi-immersed, stromatic, uni- or multi-loculate, ostiolate pycnidia, phialidic, discrete conidiogenous cells and ellipsoidal to cylindrical, hyaline, aseptate conidia, without known sexual morph.
Towyspora aestuari Wanasinghe, E.B.G. Jones & K.D. Hyde, Fungal Divers 78: 35 (2016)
Index Fungorum number: IF551788, Facesoffungi number: FoF01672; Fig. 20
Saprobic on decaying submerged twigs in freshwater habitats. Asexual morph: Conidiomata 250–400 μm high, 230–510 μm diam., scattered, pycnidial, semi-immersed to immersed, globose to subglobose, dark brown to black, uni- to biloculate, ostiolate, papillate. Conidiomatal wall 11–15 µm wide at the base, 21–24 µm wide near the ostiole, outer layers composed of thick-walled, dark brown cells of textura angularis, inner layers composed of thin-walled, hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells integrated, rarely discrete, phialidic, hyaline, cylindric-clavate to ampulliform, 6–9.5 × 2.5–5 µm (\({\overline{\text{x}}}\) = 7.8 × 4 μm, n = 20). Conidia solitary, hyaline, ellipsoidal to cylindrical, 10–13 × 2–3.5 μm (\({\overline{\text{x}}}\) = 11.5 × 2.5 μm, n = 20), with rounded or obtuse ends, 1-septate, slightly constricted at the septum, smooth and thin-walled, guttulate. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium and germ tubes produced from both ends within 12 h. Colonies on PDA medium overgrowing the culture dish after 1 month at 25 °C in dark, circular, with dense, velvety, white mycelium in the middle, becoming gray with age, umbonate in the middle; in reverse, yellowish in the middle and gray at the entire margin.
Material examined: CHINA, Guizhou Province, Bijie City, Weining, Caohai national nature reserve, 26.817° N, 104.217° E, on submerged decaying aquatic plants in Caohai Lake, L.L. Liu, 12 April 2019, 19C-19 (GZAAS 20-0385), living culture GZCC 19-0490; ibid, 19C-40 (GZAAS 20-0383), living culture GZCC 19-0488.
Notes: Based on the morphological traits and molecular evidence, our strains GZCC 19-0488 and GZCC 19-0490 are identified as T. aestuari. Towyspora aestuari was described on Phragmites communis from the UK. In this study, we report a new geographical record of T. aestuari in China and extend its habitat to freshwater.
Lindgomycetaceae K. Hiray., Kaz. Tanaka & Shearer
Notes: Lindgomycetaceae was introduced to accommodate Lindgomyces and its sister taxon Massariosphaeria typhicola (as Massariosphaeria sp. KT667 and KT 797) (Hirayama et al. 2010). Subsequently, five new genera Aquimassariosphaeria (Dong et al. 2020b), Arundellina (Hyde et al. 2016b), Hongkongmyces (Tsang et al. 2014), Lolia (Abdel-Aziz and Abdel-Wahab 2010) and Neolindgomyces (Jayasiri et al. 2019) and a previous genus Clohesyomyces (Hyde 1993; Zhang et al. 2012b) were included in Lindgomycetaceae. Species of the family are commonly saprobic on decaying stems, rachis, or wood from aquatic habitats. Some also occur in terrestrial habitats and rarely as pathogens (Hongkongmyces) associated with human infections. The family is characterized by immersed, semi-immersed, erumpent to superficial ascomata papillate or clypeus-like on the host surface, mostly with ocular chamber of asci and cylindrical, fusiform or clavate, hyaline or brown, uni- or multi-septate, straight or slightly curved ascospores with or without a mucilaginous sheath; asexual morph coelomycetous having dark brown to black or pearl white to dull yellow, ostiolate pycnidia, phialidic or holoblastic conidiogenous cells sometimes with sympodial proliferations and hyaline, cylindrical, ellipsoid, obovoid, globose to subglobose or irregular, 0–1-septate conidia with or without a mucilaginous sheath or appendages (Dong et al. 2020b).
Aquimassariosphaeria W. Dong & Doilom
Notes: Dong et al. (2020b) established Aquimassariosphaeria with the type species A. kunmingensis and a second species A. typhicola. Two strains initially identified as Massariosphaeria typhicola, CBS 609.86 and CBS123126, were reassigned as Aquimassariosphaeria typhicola and Neomassariosphaeria typhicola, respectively (Dong et al. 2020b). Aquimassariosphaeria is similar to Neomassariosphaeria in having papillate ascomata, sometimes staining the substrates purple, cylindrical-clavate asci and fusiform to vermiform ascospores. However, they were placed in Lindgomycetaceae and Amniculicolaceae, respectively, based on molecular data (Wijayawardene et al. 2018; Jayasiri et al. 2019; Dong et al. 2020b; Hongsanan et al. 2020a) (Figs. 1, 5, 21).
Aquimassariosphaeria vermiformis L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
Index Fungorum number: IF559453; Facesoffungi number: FoF12787; Fig. 22
Etymology: referring to the vermiform ascospores.
Holotype: GZAAS 20-0390
Saprobic on decaying submerged stems in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata (120–)160–210 × 190–320 μm, scattered or gregarious, immersed to semi-immersed, dark brown, globose or subglobose, papillate, ostiolate. Ostiole periphysate. Ascomatal wall 15–50 μm thick, composed of several layers of thick-walled, brown cells of textura angularis, cells becoming hyaline, thin-walled, and smaller towards the inner layers. Pseudoparaphyses abundant, cellular, hyaline, septate, branched and anastomosing, 2.5–5 µm (\({\overline{\text{x}}}\) = 3.5 μm, n = 20) wide. Asci 95–127 × 14–23 μm (\({\overline{\text{x}}}\) = 110 × 18 μm, n = 20), bitunicate, 8-spored, clavate, with a short pedicel, subapical cytoplasm occupies the whole ascus. Ascospores 25–40 × 6–8 μm (\({\overline{\text{x}}}\) = 31 × 7 μm, n = 30), obliquely 2–3-seriate, pale yellowish to yellowish brown, narrowly fusiform, straight or slightly curved, 5–8-septate, mostly 7-septate, strongly constricted at the septa, thick-walled, guttulate, smooth-walled, with an ellipsoidal to fusiform mucilaginous sheath.
Cultural characteristics: Ascospores germinating on WA medium within 24 h and germ tube produced from both ends. Colonies on PDA medium fast-growing, permeated after 20 days at 25 °C in dark, circular, with grayish white aerial mycelium on the surface, dense in the middle, sparse towards the edge; in reverse dark brown in the middle and paler at the undulate margin.
Material examined: CHINA, Guizhou Province, Bijie City, Weining, Caohai national nature reserve, 26.817° N, 104.217° E, on decaying submerged stems of aquatic plants in Caohai Lake, 4 October 2018, L.L. Liu, 19C-36 (GZAAS 20-0390, holotype), ex-type culture GZCC 19-0495; ibid, 19C-41 (GZAAS 20-0391, paratype), ex-paratype culture GZCC19-0496; ibid, 19C-42 (GZAAS 20-0392, paratype), ex-paratype culture GZCC19-0497.
Notes: In the phylogenetic analysis, Aquimassariosphaeria vermiformis grouped with A. kunmingensis (KUMCC 18-1019) and A. typhicola (CBS 609.86) with good support (92% MLBS/0.98 PP) (Fig. 21). Aquimassariosphaeria vermiformis resembles A. kunmingensis and A. typhicola with dark brown to black, papillate, ostiolate ascomata, clavate asci with short pedicel and 2–3-seriate, narrowly fusiform, straight, or slightly curved, yellowish brown, multi-septate ascospores constricted at the septa (Leuchtmann 1984; Dong et al. 2020b). Ascomata of A. kunmingensis are mostly superficial while A. vermiformis and A. typhicola are often immersed. The purple staining of the substrate was only observed in A. typhicola. Asci of A. kunmingensis [160–200 × 15–18 μm (\({\overline{\text{x}}}\) = 180 × 17 μm)] are longer but narrower than in A. vermiformis [95–127 × 14–23 μm (\({\overline{\text{x}}}\) = 110 × 18 μm)] and A. typhicola (100–160 × 15–25 μm) (Leuchtmann 1984; Dong et al. 2020b). Ascospores of the new species [25–40 × 6–8 μm (\({\overline{\text{x}}}\) = 31 × 7 μm)] are smaller than that of A. kunmingensis [35–46(–55) × 6.5–9.5 μm (\({\overline{\text{x}}}\) = 40 × 7.8 μm)] and A. typhicola (26–52 × 6–11 μm). The mucilaginous ascospore sheath is present in the new species but absent in A. kunmingensis and A. typhicola. Comparisons of the LSU, SSU, and TEF1α sequences of A. vermiformis (GZCC 19-0495) and A. kunmingensis (KUMCC 18-1019) revealed 100% (825/825 bp), 100% (820/820 bp) and 98.56% (824/836 bp) sequence similarity, respectively. The LSU and SSU sequences of A. vermiformis (GZCC 19–0495) differ from A. typhicola (CBS 609.86) by four bp (767/771 bp) and two bp (788/790 bp), respectively. The ITS sequence is unavailable for A. kunmingensis and A. typhicola. Although the molecular data shows high similarity, we recognize A. vermiformis as a new species based on their morphological differences.
Maximum likelihood majority rule consensus tree for Lindgomycetaceae using LSU, ITS, SSU, and TEF1α sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated near branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Lophiotrema eburnoides (KT 1424-1) and Lophiotrema fallopiae (HHUF 30506). The new taxa are indicated in blue and ex-type strains are indicated with T after the strain number. Genera and families are indicated with colored blocks. Branches with 100% ML BS and 1.0 PP are thickened
Hongkongmyces Tsang, Chan, Trendell-Smith, Ngan, Ling, Lau & Woo
Notes: Hongkongmyces was established based on H. pedis which was associated with human infection and formed velvety grey colonies on media without fruiting bodies or other sporulating structures (Tsang et al. 2014). Hongkongmyces aquaticus, H. kokensis and H. snookiorum are freshwater coelomycetes initially collected on submerged wood or detritus (Crous et al. 2018b; Dong et al. 2020b; Boonmee et al. 2021). Hongkongmyces brunneisporus and H. thailandica are only known as sexual morphs (Hyde et al. 2017b; Bao et al. 2021a).
Hongkongmyces aquisetosus J. Yang Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559454; Facesoffungi number: FoF12788; Fig. 23
Hongkongmyces aquisetosus (HKAS 112625, holotype) a, b Colony on wood. c Section of an ascoma. d Hyphae growing from ascomatal wall. e, f Section of peridium. g Pseudoparaphyses. h Apex of an ascus. i Stalk of an ascus. j–p Asci. q–v Ascospores (r, v with sheath). w Germinated ascospore. x, y Colony on PDA medium, x from above, y from below. Scale bars: c = 100 μm, e, f = 50 μm, d, g, j–p = 30 μm, h, i = 20 μm, q–w = 15 μm
Etymology: referring to the aquatic habitat and setose ascomata.
Holotype: HKAS 112625
Saprobic on submerged decaying wood. Asexual morph: Undetermined. Sexual morph: Ascomata 250–350 μm high, 330–370 μm diam., scattered or gregarious, semi-immersed to superficial, perithecial, subglobose, dark brown, ostiolate, papillate, covered with sparse pale brown, septate hyphae arising from ascomatal surface. Ostiole periphysate. Ascomatal wall coriaceous, 34–83 μm thick, consisting of multi-layered cells of textura angularis, outer layers with dark brown, polygonal, thick-walled cells, inner layers with pale brown to hyaline, polygonal to elongated, thin-walled cells. Hamathecium composed of compact pseudoparaphyses, 2–3 µm wide, cellular, hyaline, septate, branched. Asci (112–)135–170(–210) × 12.5–15 µm (\(\overline{\text{x}}\) = 160 × 13.5 µm, n = 20), bitunicate, fissitunicate, cylindrical to narrowly clavate, 8-spored, pedicellate, usually flexuous or slightly curved, with an apical chamber. Ascospores (23–)26–34(–37) × 6–9 µm (\(\overline{\text{x}}\) = 30 × 7.5 µm, n = 40), overlapping, obliquely 1–2-seriate, narrowly fusiform with pointed ends, straight or slightly curved, yellowish green, 0–1-septate when young, mostly 5-septate when mature, smooth-walled, guttulate, constricted at the septa, surrounded by a mucilaginous sheath.
Culture characteristics: Ascospores germinating on WA medium within 24 h and germ tubes produced from both ends. Colonies growing on PDA medium reaching 10–15 mm in 2 weeks at 25 °C in dark, circular, flat, felty, with velvety, dark olivaceous mycelium on the surface, sparse at the edge; in reverse dark olivaceous with entire margin.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying twigs submerged in Suoluo River, 19 October 2016, J. Yang, GD2-4 (HKAS 112625, holotype; HKAS 125930, isotype), ex-type cultures MFLUCC 17-0223 and GZCC 20-0357.
Notes: In the phylogenetic analysis inferred from a combined LSU-ITS-SSU-TEF1α sequence data, Hongkongmyces aquisetosus was positioned as a sister taxon to H. brunneosporus (MFLUCC 18-1509 and MFLUCC 17-1317) but with weak support (Fig. 21). Hongkongmyces aquisetosus and H. brunneosporus share the characters in having papillate, setose ascomata, cylindrical-clavate asci with an ocular chamber and short pedicel and fusiform ascospores with a mucilaginous sheath. However, H. aquisetosus has longer but narrower asci and smaller ascospores than H. brunneosporus [asci 104–125 × 19–23 µm (\(\overline{\text{x}}\) = 114 × 21 µm), ascospores 37–44 × 9–11 µm (\(\overline{\text{x}}\) = 40.3 × 10.2 µm)] (Bao et al. 2021a). Ascospores of H. aquisetosus are yellowish green and mostly 5-septate while in H. brunneosporus they are reddish brown to dark brown and mostly 7-septate. Comparisons of the LSU, SSU, and TEF1α sequence of H. aquisetosus and H. brunneosporus showed 99.65% (854/857 bp), 100% (991/991 bp) and 97% (929/954 bp) similarity, respectively. Hongkongmyces aquisetosus is distinguished from H. thailandicus which has immersed to semi-immersed, glabrous ascomata, shorter and broader asci [95–152 × 18–30 µm (\(\overline{\text{x}}\) = 123 × 25 µm)] and larger, hyaline, 1-septate ascospores [45–60 × 8–18 µm (\(\overline{\text{x}}\) = 50 × 11 µm)] (Hyde et al. 2017b). The LSU, SSU and TEF1α sequences of H. aquisetosus and H. thailandicus showed 98.29% (808/822 bp, one gap), 99.89% (974/975 bp, one gap) and 96.44% (866/898 bp) similarity, respectively.
Ocellisimilis J. Yang, L.L. Liu & K.D. Hyde, gen. nov.
Index Fungorum number: IF559455; Facesoffungi number: FoF12789
Etymology: From the Latin word, ocellus = little eye, similis = similar to, in reference to the erumpent ascomata that are similar to little eyes on the host surface.
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata immersed, perithecial, subglobose to conical, dark brown to black, papillate, ostiolate, slit-like on host surface. Ascomatal wall coriaceous, composed of thick-walled, dark brown cells of textura angularis in the outer wall layer, becoming pale brown to hyaline cells of textura angularis towards inner layers. Pseudoparaphyses numerous, cellular, hyaline, branched, septate. Asci cylindrical to clavate, rounded at the apex, 8-spored, bitunicate, short pedicellate. Ascospores clavate, golden brown, multi-septate, slightly constricted at the septa, sometimes slightly curved.
Type species: Ocellisimilis clavata L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu
Ocellisimilis clavata L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
Index Fungorum number: IF559456; Facesoffungi number: FoF12790; Fig. 24
Etymology: referring to the clavate ascospores.
Holotype: GZAAS 20-0393
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 140–195 × 178–220 μm, scattered or gregarious, immersed, perithecial, subglobose to conical, dark brown to black, ostiolate, slit-like on host surface. Ascomatal wall coriaceous, 15.5–35 μm thick, composed of thick-walled, dark brown cells of textura angularis in the outer layer, becoming pale brown to hyaline cells of textura angularis towards inner layers. Pseudoparaphyses 2–3 μm wide, numerous, cellular, hyaline, branched, septate, embedded in a matrix. Asci 82–137 × 21–30 μm (\({\overline{\text{x}}}\) = 113 × 26 μm, n = 20), cylindrical to clavate, rounded at the apex, 8-spored, bitunicate, short pedicellate. Ascospores 31–40 × 7–10 μm (\({\overline{\text{x}}}\) = 35 × 10 μm, n = 30), partially overlapping, arranged obliquely 2–3-seriate, clavate, broadest at the third cell from the apex, hyaline to pale yellow when young, dark yellow to yellowish brown when mature, 7–10-septate, slightly constricted at the septa, sometimes slightly curved.
Cultural characteristics: Ascospores germinating on WA medium within 24 h. Germ tubes can produce from every cell. Colonies on PDA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in dark, circular, with dense, velvety, grayish brown mycelium on the surface; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Bijie City, Weining, Caohai national nature reserve, 26.817° N, 104.217° E, on submerged decaying twigs in Caohai Lake, L.L. Liu, 4 October 2018, 18C-21 (GZAAS 20-0393, holotype), ex-type culture GZCC 19-0498; ibid, 18C-27 (GZAAS 20-0394, paratype), ex-paratype culture GZCC 19-0499.
Notes: Two strains of Ocellisimilis clavata (GZCC 19-0498 and GZCC 19-0499) were nested within Lindgomycetaceae (Fig. 21) and are described here as a new genus Ocellisimilis. Ocellisimilis formed a distinct clade and differs from other genera in the family. Ocellisimilis clavata is clearly distinguished from other species in Lindgomycetaceae by its immersed ascomata, slit-like on the host surface and clavate, golden brown ascospores. Sexual taxa in Lindgomycetaceae mostly possess hyaline or brown, fusiform ascospores, except Lolia dictyospora which resembles Ocellisimilis clavata by clavate ascospores. However, ascospores of Lolia dictyospora are yellowish brown to dark brown, with 0–1 longitudinal or oblique septa, 3–6 transverse septa and surrounded by a conspicuous mucilaginous sheath, while that are golden brown, 7–10-transverse-septate, without a sheath in the new species (Abdel-Aziz 2016).
Longipedicellataceae Phukhams., J. Bhat & K.D. Hyde
Notes: Longipedicellataceae was established to accommodate Longipedicellata and Pseudoxylomyces based on molecular evidence and divergence time estimation (Phukhamsakda et al. 2016). A new genus Submersispora was recently introduced to the family (Dong et al. 2020b). The family members are saprobic on submerged bamboo or twigs from aquatic habitats (Goh et al. 1997b; Tanaka et al. 2015; Phukhamsakda et al. 2016; Zhang et al. 2016; Dong et al. 2020b). Longipedicellata is known as sexual morphs producing moniliform chlamydospores in culture, while Pseudoxylomyces and Submersispora are characterized by brown fusiform and dark brown variable-shaped conidia, respectively, with no known sexual morphs.
Longipedicellata H. Zhang, K.D. Hyde & J.K. Liu
Notes: Longipedicellata was introduced to accommodate Didymella aptrootii which is commonly found on submerged bamboo or wood in freshwater habitats (Zhang et al. 2016). Currently, three species are accepted in the genus (Chethana et al. 2021; Index Fungorum 2022).
Longipedicellata aquatica W. Dong, H. Zhang & K.D. Hyde, Fungal Divers 105: 435 (2020)
Index Fungorum number: IF557915; Facesoffungi number: FoF09257; Fig. 25
Longipedicellata aquatica (MFLU 15-1165) a–c Colonies on substrate. d, e Section of ascomata. f Section of peridium. g Pseudoparaphyses. h–n Asci. o Apex of an ascus. p–s Ascospores. t Germinated ascospore on PDA medium. u–v Culture, u from above, v from reverse. Scale bars: a = 400 μm, b, c = 100 μm, l = 50 μm, d, e = 40 μm, h, k = 30 μm, i, j, m, n, t = 20 μm, g, o = 15 μm, p–s = 10 μm, f = 5 μm
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 90–195 μm high, 110–240 μm wide, scattered or gregarious, semi-immersed to superficial, erumpent, perithecial, globose to subglobose, dark brown, ostiolate. Ostiole periphysate. Ascomatal wall coriaceous, 15–27.5 μm thick, consisting of multi-layered cells of textura angularis, outer layers with dark reddish brown, polygonal, thick-walled cells, inner layers with pale brown to hyaline, polygonal to elongated, thin-walled cells. basal cells undifferentiated from host tissue. Hamathecium composed of compact pseudoparaphyses, 2–3.3 µm wide, cylindrical, hyaline, septate, branched. Asci 76–115(–155) × (19–)24–29(–32) µm (\({\overline{\text{x}}}\) = 100 × 25 µm, n = 20), bitunicate, fissitunicate, cylindrical or clavate, 8-spored, elongate when released in water, up to 200 μm long, with a long, straight or twisted pedicel and an apical chamber. Ascospores (16–)19–23(–27) × (7–)8.5–11(–13) µm (\({\overline{\text{x}}}\) = 21 × 9.5 µm, n = 35), overlapping, obliquely 2–3-seriate, broadly fusiform with rounded ends, straight or slightly curved, hyaline when young, becoming mid brown with age, 1-septate, smooth-walled, guttulate, deeply constricted at the septum, apical cell often slightly broader than the basal cell.
Cultural characteristics: Ascospores germinating on PDA medium within 24 h and germ tubes produced from both ends. Colonies on MEA medium reaching 5–10 mm diam. in a week at 25 °C, in natural light, circular, with fluffy, dense, dark grayish green mycelium on the surface; in reverse, dark brown to black with entire margin.
Material examined: THAILAND, Prachuap Khiri Khan Province, 12.503° N, 99.523° E, on decaying wood submerged in a freshwater stream, 25 December 2014, J. van Strien, site5-10-1 (MFLU 15-1165), living culture MFLUCC 15-0630; ibid, site5-15-1 (HKAS 112176), living culture MFLUCC 16-0182.
Notes: In the phylogenetic analysis, our collections grouped with Longipedicellata aquatica (MFLUCC 19-0324 and MFLUCC 17-2334) (Fig. 1). The ITS sequences of our collections showed 23 bp (511/534 bp), two bp (487/489 bp) and two bp (544/546 bp) differences from L. aptrootii (MFLUCC 10-0297, reference sequence), L. aquatica (MFLUCC 17-2334, ex-type) and L. megafusiformis (MFLUCC 21-0085, ex-type), respectively. Our collections have high sequence similarity with L. aquatica and L. megafusiformis of the ITS gene region. Their phylogenetic relationship cannot be confirmed as protein-coding gene is unavailable for the ex-type of L. aquatica. However, our collection matches well with the morphology and dimensions of L. aquatica (Dong et al. 2020b). We therefore identify our collections as L. aquatica. In this study, L. aquatica was recollected from Thailand and its ascospores became brown when old which was not observed in the protologue (Dong et al. 2020b).
Pseudoxylomyces Kaz. Tanaka & K. Hiray.
Notes: Pseudoxylomyces comprises two species that are saprobic on submerged wood in freshwater habitats. The genus was segregated from Xylomyces by the broadly fusiform conidia and its systematic placement (Tanaka et al. 2015).
Pseudoxylomyces elegans (Goh, W.H. Ho, K.D. Hyde & C.K.M. Tsui) Kaz. Tanaka & K. Hiray., Stud Mycol 82: 126 (2015)
Index Fungorum number: IF811333; Facesoffungi number: FoF09259, Fig. 26
Basionym: Xylomyces elegans Goh, W.H. Ho, K.D. Hyde & C.K.M. Tsui, Mycol Res 101(11): 1324 (1997)
Saprobic on submerged decaying wood in freshwater habitats. Asexual morph: Colonies on wood effuse, scattered or aggregated, brown, glistening, conidia arising from aerial hyphae. Aerial hyphae smooth, septate, sparsely branched, hyaline to pale brown, thin-walled, 1.8–4 µm wide. Conidiogenous cells monoblastic, integrated. Conidia broadly fusiform, tapering towards both ends, 4–8-septate, smooth-walled, mid brown, 72–110 × 24–29 µm (\({\overline{\text{x}}}\) = 92.5 × 27 µm, n = 30), guttulate, strongly constricted at the septa, paler at the ends. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h and germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm in 2 weeks at 25 °C in natural light, circular, with velvety, grayish brown and white mycelium on the ring-like surface; in reverse dark brown in the middle, white and smooth at the entire margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT30-3 (MFLU 22-0073 and HKAS 112162), living cultures MFLUCC 17-2132 and GZCC 20-0406.
Notes: Pseudoxylomyces elegans is a widespread species and was recollected in Thailand in this study. The phylogenetic placement of our collection is shown in Fig. 1.
Morosphaeriaceae Suetrong, Sakayaroj, E.B.G. Jones & C.L. Schoch
Notes: Morosphaeriaceae was established by Suetrong et al. (2009) containing Helicascus, Morosphaeria and Kirschsteiniothelia elaterascus with strong molecular support. Presently, six genera are accepted in the family, Aquihelicascus (Dong et al. 2020b), Aquilomyces (Knapp et al. 2015), Clypeoloculus (Tanaka et al. 2015), Helicascus (Kohlmeyer 1969; Dong et al. 2020b), Morosphaeria (Suetrong et al. 2009) and Neohelicascus (Dong et al. 2020b). Members in Morosphaeriaceae are mainly saprobic on roots or twigs of mangroves in marine habitats or submerged twigs in freshwater habitats; only Aquilomyces patris is a root endophyte of white poplar.
Aquihelicascus W. Dong, H. Zhang & Doilom
Notes: Based on the morphological characters of asci and ascospores and molecular evidence, Helicascus was divided into three groups: the core with the type species and two monoclades representing the new genera Aquihelicascus and Neohelicascus, respectively (Dong et al. 2020b). Helicascus comprises three species, H. kanaloanus, H. mangrovei, H. nypae, characterized by semi-immersed or immersed, carbonaceous, ostiolate ascomata enclosing single or multi locules that are horizontally arranged under a black pseudoclypeus composed of host tissue, subcylindrical, pedicellate asci with an ocular chamber and coiled endoascus at the basal part, and uniseriate, obovoid, brown, asymmetrical, uniseptate ascospores with one or both ends apiculate, mucilaginous sheath present or absent (Kohlmeyer 1969; Hyde 1991; Preedanon et al. 2017).
Aquihelicascus was introduced to accommodate two new species A. songkhlaensis and A. yunnanensis and a new combination A. thalassioideus (synonym: Helicascus thalassioideus). Aquihelicascus is distinguished from Helicascus by clavate asci with uncoiled endoascus, and biseriate, ellipsoidal, symmetrical, hyaline ascospores with rounded ends (Dong et al. 2020b).
Aquihelicascus thalassioideus (K.D. Hyde & Aptroot) W. Dong & H. Zhang, Fungal Divers 105: 463 (2020)
Index Fungorum number: IF557920; Facesoffungi number: FoF09262; Fig. 27
Aquihelicascus thalassioideus (MFLU 22-0062) a Colony on wood. b, c Section of ascomata. d, e Section of peridium. f Pseudoparaphyses. g–n Asci. o, p Ascospores. q Germinated ascospore. r, s Colony on MEA medium, r from above, s from below. Scale bars: a = 500 μm, b = 200 μm, c = 100 μm, g–n = 30 μm, d–f, q = 20 μm, o, p = 15 μm
Basionym: Massarina thalassioidea K.D. Hyde & Aptroot, Nova Hedwig 66: 498 (1998)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 250–300 μm high, 120–330 μm diam., scattered or gregarious, immersed, perithecial, subglobose, dark brown, ostiolate, with masses of ascospores at the apex of the immersed to superficial neck. Ostioles periphysate. Ascomatal wall coriaceous, 13–25 μm thick, undifferentiated from the host tissue, consisting of multi-layered cells of textura angularis, outer layers with brown, polygonal, thick-walled cells, inner layers with pale brown to hyaline, polygonal to elongated, thin-walled cells. Hamathecium composed of 1–2 µm wide, filiform, hyaline, septate pseudoparaphyses, embedded in a gel matrix. Asci 103–128 × 14–20 µm (\({\overline{\text{x}}}\) = 114 × 17 µm, n = 20), bitunicate, fissitunicate, clavate, rounded at the apex with an ocular chamber, 8-spored, usually with a short pedicel. Ascospores 25–29 × 8–11 µm (\({\overline{\text{x}}}\) = 27 × 9.5 µm, n = 30), overlapping, obliquely biseriate, fusiform, hyaline, uniseptate, smooth-walled, guttulate, slightly constricted at the septum, apical cell usually slightly broader and longer than the basal cell.
Culture characteristics: Ascospores germinating on PDA medium within 24 h and germ tubes produced from both ends. Colonies on MEA medium, reaching 10–15 mm in 2 weeks at 25 °C in natural light, circular, with dense, velvety, reddish brown mycelium on the surface; in reverse dark reddish brown in the middle, paler at the entire margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT2-2 (MFLU 22-0062 and HKAS 112148), living cultures MFLUCC 17-2048 and GZCC 20-0369.
Notes: Aquihelicascus thalassioideus is a widespread species found commonly on submerged wood in freshwater habitats (Tanaka et al. 2015). In this study, we re-collected this species in Thailand. The phylogenetic placement of our collection is shown in Fig. 1.
Neohelicascus W. Dong, H. Zhang, K.D. Hyde & Doilom
Notes: Neohelicascus comprises eight species with seven transferred from Helicascus. The genus shares the coiled endoascus at the basal part with Helicascus but differs by biseriate, straight or slightly curved, ellipsoidal to fusiform ascospores with rounded ends (Dong et al. 2020b). A coelomycetous asexual morph was reported for Neohelicascus (Zhang et al. 2013).
Neohelicascus chiangraiensis (Z.L. Luo, J.K. Liu, H.Y. Su & K.D. Hyde) W. Dong, K.D. Hyde & H. Zhang, Fungal Divers 105: 466 (2020)
Index Fungorum number: IF557923; Facesoffungi number: FoF09265; Fig. 28
Basionym: Helicascus chiangraiensis Z.L. Luo, J.K Liu, H.Y. Su & K.D. Hyde, Phytotaxa 270(3): 185 (2016)
Saprobic on decaying submerged twigs in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 300–500 μm high, 300–700 μm diam., solitary, scattered or aggregated, immersed, unilocular or multilocular, globose to subglobose, ostiolate. Ostioles periphysate. Ascomatal wall (37.5–)51–85.5 μm (\({\overline{\text{x}}}\) = 63.5 μm, n = 15) thick, composed of dark brown, thick-walled cells of textura angularis in outer layers, becoming pale brown to hyaline in inner layers. Hamathecium composed of hypha-like, 1.5–2.5 μm wide pseudoparaphyses, slightly constricted at the septa, longer than asci, ramified above asci with free ends, embedded in a gel matrix. Asci 74–128 × 17–24 μm (\({\overline{\text{x}}}\) = 106 × 20 μm, n = 20), bitunicate, fissitunicate, 8-spored, clavate, apically rounded, dehiscence, endoascus narrow, coiled within ectoascus, ectoascus forming a long tail extension. Ascospores 22–26 × 5–10 μm (\({\overline{\text{x}}}\) = 24.5 × 7 μm, n = 30), biseriate, fusiform, straight or slightly curved, pale yellowish when young, becoming dark yellowish brown when mature, uniseptate, slightly constricted at the septum, thick-walled, verruculose, guttulate, surrounded by a fusiform mucilaginous sheath.
Cultural characteristics: Ascospores germinating on WA medium within 24 h and germ tube produced from both ends. Colonies on PDA medium reaching 10–15 mm diam. after 3 weeks at 25 °C in dark, circular, with dense, velvety, white mycelium on the surface, becoming yellow with age; in reverse dark brown in the middle, white at the entire margin.
Material examined: CHINA, Guizhou Province, Guiyang City, Aha Lake, 26.533° N, 106.667° E, on decaying branch submerged in a lake, 16 April 2018, L.L. Liu, 18A-10 (GZAAS 20-0328), living culture GZCC 19-0433.
Notes: Our specimen matches with Neohelicascus chiangraiensis based on the original description of the holotype given by Luo et al. (2016a), though the ascospores are slightly smaller. Neohelicascus chiangraiensis is known on dead decaying wood submerged in freshwater habitats in China and Thailand (Luo et al. 2016a; Brahmanage et al. 2020). In this study, the fungus was recollected in China. The phylogenetic placement of our collection is shown in Fig. 1.
Neohelicascus gallicus (Y. Zhang & J. Fourn) W. Dong, K.D. Hyde & H. Zhang, Fungal Divers 105: 468 (2020)
Index Fungorum number: IF557926; Facesoffungi number: FoF09268; Fig. 29
Neohelicascus gallicus (HKAS 112642) a, b Colony on wood. c, d Section of ascomata. e Section of peridium. f Pseudoparaphyses. g–m Asci. n–q Ascospores. r Apex of an ascus. s Germinated ascospore. t, u Colony on PDA medium, t from above, u from below. Scale bars: c = 200 μm, d = 100 μm, e–n = 30 μm, o–s = 15 μm
Basionym: Helicascus gallicus Y. Zhang & J. Fourn., Phytotaxa 183: 185 (2014)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 250–450 μm high, 180–380 μm diam., scattered or gregarious, immersed, perithecial, subglobose or obovoid, dark brown, ostiolate, with masses of ascospores at the apex of the immersed to superficial long neck. Ostioles periphysate. Ascomatal wall coriaceous, 26–45 μm thick, thickened around the ostiole, consisting of multi-layered cells of textura angularis, outer layers with brown, polygonal, thick-walled cells, inner layers with pale brown to hyaline, polygonal to elongated, thin-walled cells. Hamathecium composed of 1.9–3 µm wide, filiform, hyaline, septate pseudoparaphyses, embedded in a gel matrix. Asci 88–175 × 16–24 µm (\({\overline{\text{x}}}\) = 130 × 19 µm, n = 20), bitunicate, fissitunicate, clavate, rounded at the apex with an ocular chamber, 8-spored, with a long pedicel. Ascospores 21–28 × 6.5–11 µm (\({\overline{\text{x}}}\) = 24 × 8.6 µm, n = 30), overlapping, obliquely biseriate, fusiform to lunate, dark olivaceous brown, uniseptate, smooth-walled, guttulate, slightly constricted at the septum, apical cell usually slightly broader and longer than the basal cell, with a mucilaginous sheath.
Cultural characteristics: Ascospores germinating on WA medium within 24 h and germ tube produced from one or both ends. Colonies on PDA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in dark, circular, with brown to dark brown mycelium on the surface, dense in the middle and sparse at the edge, in reverse dark brown to black with pale brown and entire margin.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying wood submerged in Suoluo River, 17 October 2018, J. Yang, GDT19-1 (HKAS 124638), living cultures MFLUCC 19-0298 and GZCC 20-0478; CHINA. Guizhou Province, Anshun City, on decaying wood submerged in a freshwater stream, 19 October 2016, J. Yang, PZ-4 (HKAS 112642), living cultures MFLUCC 17-0239 and GZCC 20-0367.
Notes: Our collections fit the morphology of Neohelicascus gallicus with slightly smaller asci (Zhang et al. 2014b). The type material of N. gallicus possesses immersed pseudostromata comprising 2–3 locules beneath a common clypeus, flush with the surface or raised into a dome-shaped structure. However, our collection has mostly immersed unilocular ascomata with a cylindrical neck on the host surface. The LSU and ITS sequences of our collections showed 100% and 99.81% (517/518 bp) similarity with the holotype of N. gallicus. We therefore recognize our collections as N. gallicus and report as a new geographical record of this species in China and Thailand, respectively. The phylogenetic placement of our collections is shown in Fig. 1.
Neohelicascus griseoflavus J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF559602; Facesoffungi number: FoF12791; Fig. 30
Neohelicascus griseoflavus (MFLU 22-0059, holotype) a Colony on wood. b Section of ascomata. c, d Section of peridium. e–i Asci. j Pseudoparaphyses. k–n Ascospores. o Germinated ascospore. p, q Colony on MEA medium, p from above, q from below. Scale bars: a = 1000 μm, b = 200 μm, f–i, k, o = 30 μm, c–e, j, l–n = 20 μm
Etymology: grisei = gray, flavus = yellow, referring to the grayish yellow ascospores.
Holotype: MFLU 22-0059
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 280–400 μm high, 250–380 μm diam., scattered or gregarious, immersed, perithecial, subglobose, brown, ostiolate, with masses of ascospores at the apex of the immersed short neck. Ostioles periphysate. Ascomatal wall coriaceous, undifferentiated from host tissue, 20–50 μm thick, consisting of multi-layered cells of textura angularis, outer layers with pale brown, polygonal, thick-walled cells, inner layers with pale brown to hyaline, polygonal to elongated, thin-walled cells. Hamathecium composed of 1–2.5 µm wide, filiform, hyaline, septate pseudoparaphyses. Asci (120–)150–200(–215) × (15–)19–22(–24) µm (\(\overline{\text{x}}\) = 170 × 20 µm, n = 20), bitunicate, fissitunicate, 8-spored, clavate, rounded at the apex with an ocular chamber, pedicellate, with a long tail-like extension. Ascospores 24–31 × 8–11 µm (\(\overline{\text{x}}\) = 28 × 10 µm, n = 30), overlapping, obliquely 1–2-seriate, lunate to fusiform, grayish yellow when young, becoming pale brown when mature, uniseptate, smooth-walled, guttulate, slightly constricted at the septum, apical cell usually slightly broader than the basal cell.
Culture characteristics: Ascospores germinating on PDA medium within 24 h. Germ tubes produced from one or both ends. Colonies on MEA medium reaching 10 mm diam. after 1 week at 25 °C in natural light, circular, with dense, grayish white and grayish green mycelium on the surface, in reverse dark green with paler and entire margin.
Material examined: THAILAND, Phang Nga Province, Bann Tom Thong Khang, on decaying wood submerged in a freshwater stream, 17 December 2015, J. Yang, site7-28-1 (MFLU 22-0059, holotype; HKAS 112182, isotype); ex-type cultures MFLUCC 16-0869 and GZCC 17-0024.
Notes: In the phylogenetic analysis, Neohelicascus griseoflavus formed a distinct branch within Neohelicascus with strong support (100% MLBS/0.99 PP) (Fig. 1). It can be distinguished from other species in the genus by the pale grayish yellow ascospores becoming pale brown after release. The immersed and unilocular ascomata of N. griseoflavus resembles N. chiangraiensis, N. submersus, N. unilocularis and N. uniseptatus, but differs by mostly immersed, melanized ascomata with a relatively long neck and pale brown ascomatal wall (Zhang et al. 2015; Luo et al. 2016a; Dong et al. 2020b). However, the generic character of coiled filiform basal extension of the endoascus was not observed in our collection. Additional specimens will be needed to examine this character in N. griseoflavus. The ITS sequence of N. griseoflavus showed 88.86% (383/431 bp, 13 gaps), 90.24% (416/461 bp, 11 gaps), 90.24% (416/461 bp, 11 gaps) and 88.49% (392/443 bp, 12 gaps) sequence similarity with the type material of N. chiangraiensis, N. submersus, N. unilocularis and N. uniseptatus, respectively.
Parabambusicolaceae Kaz. Tanaka & K. Hiray.
Notes: Parabambusicolaceae comprises nine genera characterized by immersed to erumpent, ostiolate, papillate ascomata, clavate to broadly cylindrical, pedicellate asci with an ocular chamber and clavate, ellipsoidal to fusiform, hyaline, pale or reddish-brown, 1- to multi-septate ascospores with a gelatinous sheath, asexual morphs phoma-like coelomycetous or monodictys-like hyphomycetous (Tanaka et al. 2015; Hongsanan et al. 2020a). Members in the family are saprobic on dead decaying wood or bamboo in terrestrial or freshwater habitats.
Parabambusicola Kaz. Tanaka & K. Hiray.
Notes: Parabambusicola was segregated from Massarina with a single species M. bambusina (as P. bambusina) (Tanaka et al. 2015). Later, Parabambusicola thysanolaenae and P. aquatica were introduced to the genus (Phookamsak et al. 2019; Dong et al. 2020b).
Parabambusicola bambusina (Teng) Kaz. Tanaka & K. Hiray., Stud Mycol 82: 116 (2015)
Index Fungorum number: IF811392; Facesoffungi number: FoF11623; Fig. 31
Basionym: Massarina bambusina Teng, Sinensia 7: 512 (1936)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 198–302 μm high, 325–430 μm wide, mostly grouped, immersed to semi-immersed, subglobose, ostiolate, papillate. Ascomatal wall 15–30 μm thick, composed of dark brown, thick-walled, polygonal cells of textura angularis, poorly developed at the base. Pseudoparaphyses numerous, 1–2.5 μm (\({\overline{\text{x}}}\) = 1.6 μm, n = 20) wide. Asci 82–161 × 33–47 μm (\({\overline{\text{x}}}\) = 126 × 38 μm, n = 20), 8-spored, broadly cylindrical to clavate, short stalked. Ascospores 50–62 × 8–14 μm (\({\overline{\text{x}}}\) = 55 × 12 μm, n = 20), fusiform, hyaline, 3–5-septate, mostly 4-septate, slightly constricted at the septa, guttulate, smooth-walled, with a narrowly mucilaginous sheath.
Cultural characteristics: Ascospores germinating on WA medium and germ tubes produced from both ends within 12 h. Colonies growing on PDA medium and reaching 20 mm diam. after 1 month at 25 °C in dark, circular, consisting of a matted felt with velutinous appearance, grayish white when young, becoming yellowish green in the middle; in reverse yellowish to grayish green with an entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, 28.417° N, 106° E, on submerged decaying wood in a freshwater stream, July 2019, L.L. Liu, CS1-3-2 (GZAAS 20-0400), living culture GZCC 19-0505.
Notes: Massarina bambusina was introduced by Teng (1936) that was collected on bamboo in China. It was later recollected by Tanaka and Harada (2003) on culms of Sasa kurilensis in Japan. They also re-examined the type material of Metasphaeria tuberculosa, and considered it as a synonym of Massarina bambusina. Based on more collections and further molecular evidence, Tanaka et al. (2015) introduced a new genus Parabambusicola to accommodate Massarina bambusina as Parabambusicola bambusina. The morphology of our collection matches well with the type material and additional specimens, although the asci and ascospores are slightly wider. Based on the molecular DNA data, we recognize our collection as Parabambusicola bambusina (Fig. 1). In this study, we recollected Parabambusicola bambusina in China and report a new habitat for the species from a freshwater stream.
Phaeoseptaceae S. Boonmee, Thambugala & K.D. Hyde
Notes: Phaeoseptaceae was established to accommodate Lignosphaeria, Neolophiostoma, Phaeoseptum, Decaisnella formosa and Thyridaria macrostomoides (Hyde et al. 2018). Liu et al. (2019b) introduced a new genus Pleopunctum in the family and excluded Neolophiostoma which was positioned within Halotthiaceae.
Pleopunctum N.G. Liu, K.D. Hyde & J.K. Liu.
Notes: Liu et al. (2019b) introduced Pleopunctum with two species with sporodochial conidiomata and brown, muriform, ellipsoidal conidia. Later, Pleopunctum clematidis and P. thailandicum were described in the genus by Phukhamsakda et al. (2020) and Boonmee et al. (2021), respectively.
Pleopunctum ellipsoideum N.G. Liu, K.D. Hyde & J.K. Liu, Mycosphere 10(1): 767 (2019)
Index Fungorum: IF556523; Facesoffungi number: FoF06114; Fig. 32
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates sporodochial, superficial, dark brown to black, scattered, punctiform. Mycelium partly immersed, composed of septate, branched hyphae. Conidiophores macronematous, mononematous, branched, septate, pale brown, smooth-walled, thick-walled, 1–3 μm wide (\({\overline{\text{x}}}\) = 2.5 μm, n = 15). Conidiogenous cells monoblastic, terminal, integrated, pale brown. Conidia acrogenous, solitary, ellipsoidal, olivaceous brown, darkened at the upper part, paler at the base, muriform, constricted at the septa, 36–50 × 16–20 μm (\({\overline{\text{x}}}\) = 43 × 18 μm, n = 30), smooth-walled, broadly obtuse at the apex, truncate at the base, often with a hyaline, globose to subglobose basal cell, 8–14 × 8.5–16 μm (\({\overline{\text{x}}}\) = 11.5–12 μm, n = 20). Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium and germ tubes produced from the basal cell within 12 h. Colonies on PDA medium reaching about 20–30 mm diam. after 1 month at 25 °C in dark, circular, with dense, grayish green mycelium in the middle and sparse, white mycelium at the edge, producing slimy secretions on the surface; in reverse, dark brown in the middle, white at the entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS27-2 (HKAS 112609), living culture GZCC 20-0502; CHINA, Guizhou Province, Dushan District, 25.917° N, 107.617° E, on decaying branch submerged in a freshwater stream, 5 July 2018, L.L. Liu, 18D-50 (GZAAS 20-0401), living culture GZCC 19-0506.
Notes: In the phylogenetic analysis (Fig. 1), our isolates (GZCC 20-0502 and GZCC 19-0506) clustered as sister taxa to the type material of Pleopunctum ellipsoideum (MFLUCC 19-0390). Sequence similarity of the LSU, ITS, and TEF1α gene regions between GZCC 20-0502 and the type material is 99.88% (822/833 bp), 99.22% (512/516 bp) and 98.21% (933/950 bp), respectively. Our collections’ morphology match well with the holotype of Pleopunctum ellipsoideum (Liu et al. 2019b). We therefore recognize our collections as Pleopunctum ellipsoideum based on the morphology and molecular evidence. Our specimens were collected in Guizhou Province, China, same as the holotype. However, this is the first report of Pleopunctum ellipsoideum from freshwater habitat.
Pleosporaceae Nitschke
Notes: Pleosporaceae comprises saprobic, pathogenic, parasitic and endophytic species associated with a wide variety of substrates mainly from terrestrial and aquatic habitats (Hongsanan et al. 2020a). Traditional classification based on a few characters caused taxonomic confusion of Pleosporaceae and its internal genera. Therefore, reclassifications of Pleosporaceae taxa have been frequently proposed (Kodsueb et al. 2006b). With increasing samplings and availability of molecular data, a more robust and natural taxonomy of Pleosporaceae taxa was delineated (Kodsueb et al. 2006b; Ariyawansa et al. 2015b; Hongsanan et al. 2020a). Twenty-three genera are currently accepted in the family (Hongsanan et al. 2020a).
Alternaria Nees
Notes: For a review of the genus, see Lawrence et al. (2016).
Alternaria scirpivora (E.G. Simmons & D.A. Johnson) Woudenb. & Crous, Stud Mycol 75: 198 (2013)
Index Fungorum number: IF803702; Facesoffungi number: FoF12792; Fig. 34
Basionym: Nimbya scirpivora E.G. Simmons & D.A. Johnson, Mycotaxon 84: 424 (2002)
Synonym: Macrospora scirpivora E.G. Simmons & D.A. Johnson, Mycotaxon 84: 422 (2002)
Saprobic on decaying submerged twigs in freshwater habitats. Asexual morph: Colonies effuse, hyaline, hairy, velvety. Mycelium mostly immersed, consisting of branched, septate, smooth, hyaline hyphae. Conidiophores macronematous, mononematous, erect, solitary or in fascicles, pale brown, becoming paler towards the apex, 2–7-septate, branched, smooth-walled, cylindrical, 20–74 × 4–9 μm (\({\overline{\text{x}}}\) = 45 × 6 μm, n = 20). Conidiogenous cells polytretic, integrated, terminal or intercalary, determinate, subhyaline to pale brown, cylindrical. Conidia acropleurogenous, solitary, pale yellowish brown, obclavate, rostrate, straight, or slightly curved, up to 14-septate, tapering towards the rounded apex, truncate at the base, 43–152 × 8–16 μm (\({\overline{\text{x}}}\) = 109.5 × 12 μm, n = 20), constricted at the septa, smooth-walled. Sexual morph: Ascomata 140–245 μm high, 110–290 diam., solitary or clustered, immersed, subglobose, dark brown to black, coriaceous, ostiolate. Peridium 27–66 μm (\({\overline{\text{x}}}\) = 42 μm, n = 10) thick, outer layers composed of dark brown, thick-walled cells of textura angularis, inner layers composed of pale brown to hyaline, thin-walled cells of textura angularis. Hamathecium composed of 3–8 μm wide, cellular, septate, pseudoparaphyses branching and anastomosing above asci. Asci 143–237 × 42–56 μm (\({\overline{\text{x}}}\) = 176.5 × 48 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly clavate, pedicellate. Ascospores 37–58 × 16–23 μm (\({\overline{\text{x}}}\) = 46 × 19 μm, n = 30), overlapping 2–3-seriate, muriform, ellipsoid, 5-transseptate, constricted at the septa, with one central longitudinal septum, hyaline when young, dilute yellowish at maturity, guttulate, smooth-walled.
Cultural characteristics: Conidia germinating on WA medium within 24 h and germ tube produced from both ends. Colonies on PDA medium reaching about 20–25 mm diam. after 1 week at 25 °C in dark, circular, with dense, velvety, grayish green mycelium in the middle, white at the edge; in reverse dark green in the middle, white at the entire margin.
Material examined: CHINA, Guizhou Province, Bijie City, Weining, Caohai national nature reserve, 26.817° N, 104.217° E, on stems of submerged decaying aquatic plants in Caohai Lake, October 2018, L.L. Liu, 18C-1 (GZAAS 20-0365), living culture GZCC 19-0470; ibid, April 2019, L.L. Liu, 19C-11 (GZAAS 20-0368), living culture GZCC 19-0473.
Notes: Macrospora scirpivora was initially known as a sexual morph in vitro from sporulation of the asexual morph Nimbya scirpivora isolated from stem lesions of Scirpus specimens in North America (Johnson et al. 2002). They were redefined as Alternaria scirpivora, a member of the section Nimbya in Alternaria (Woudenberg et al. 2013). Alternaria scirpivora resembles A. scirpinfestans, possessing broadly clavate asci, ellipsoidal, hyaline, muriform ascospores, macronematous, mononematous, branched conidiophores, mono- or polytretic, terminal and intercalary conidiogenous cells and rostrate, narrow, elongate conidia. They can be distinguished by the dimensions of ascospores and conidia, conidiophore development and conidial extension (Johnson et al. 2002). Besides, ascospores in the former have five transverse septa, rarely six, while the latter often reaches 6–7. In this study, we collected both asexual and sexual morphs of an Alternaria (section Nimbya) species on different decaying woody specimens. It is similar to A. scirpivora in the morphology of asci, ascospores, conidiophores and conidia with comparable dimensions. However, the conidial chain was not observed in our specimens. In the LSU-ITS-SSU-RPB2 phylogenetic tree, our collections clustered with A. scirpivora (IRAN 3513C and EGS 50-021) with strong statistical support (Fig. 33). The ITS sequences of our specimens are identical to the type material of A. scirpivora. Therefore, we recognize our collections as A. scirpivora, extend its habitat to freshwater, and report a new geographical record in China (Fig. 34). Because the barcoding gene ITS is not informative for many Alternaria species, further phylogenetic studies are required focusing on informative loci on plasma membrane ATPase and calmodulin as recommended by Lawrence et al. (2016).
Maximum likelihood majority rule consensus tree for Pleosporaceae using LSU, ITS, SSU, and RPB2 sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated near branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Neophaeosphaeria filamentosa (CBS 102202). The new collections are indicated in bold and ex-type strains are indicated with T after the strain number. Genera are indicated with colored blocks. Branches with 100% ML BS and 1.0 PP are thickened
Alternaria scirpivora (a–k GZAAS 20-0365, l–t GZAAS 20-0368) a, l Colony on wood. b Section of an ascoma. c–e Asci. f Pseudoparaphyses. g–j Ascospores. k Germinated ascospore. m, n Conidiophores. o–q Conidia. r Germinated conidium. s, t Culture, s from above, t from below. Scale bars: b–e = 50 μm, f–k, m–r = 30 μm
Pseudoastrosphaeriellaceae Phookamsak & K.D. Hyde
Notes: Pseudoastrosphaeriellaceae was established to accommodate Pseudoastrosphaeriella (Phookamsak et al. 2015b). Later, Carinispora (Hyde 1992b; Hyde et al. 2017b) and Pseudoastrosphaeriellopsis (Phookamsak et al. 2019) were accepted in the family.
Pseudoastrosphaeriella Phookamsak, Z.L. Luo & K.D. Hyde
Notes: For morphological characters and phylogenetic treatment of the genus, see Dong et al. (2020b).
Pseudoastrosphaeriella bambusae Phookamsak & K.D. Hyde, Fungal Divers 74: 186 (2015)
Index Fungorum number: IF551644, Facesoffungi number: FoF01236, Fig. 35
Pseudoastrosphaeriella bambusae (GZAAS 22-2202) a Colony on wood. b, c Vertical section of ascomata. d, e Section of peridium. f Pseudoparaphyses. g, n–s Ascospores. h–m Asci. t Germinated ascospore. u, v Colony on PDA medium, u from above, v from below. Scale bars: a = 1000 μm, b = 500 μm, c = 200 μm, f = 50 μm, d, e, g–m, t = 30 μm, n–s = 20 μm
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 258–476 μm high, 277–620 μm diam., dark brown to black, scattered, or gregarious, immersed, subglobose to lenticular, or depressed conical, with flattened base, uniloculate, glabrous, coriaceous, ostiolate, with a short neck. Ostioles periphysate. Ascomatal wall 25–80 μm thick, thickened at the sides, especially towards the apex, composed of thick-walled, dark reddish-brown cells of textura angularis in the outer layers, becoming pale brown to hyaline cells of textura angularis towards inner layers. Hamathecium composed of dense, 1–2 μm wide, filiform, trabeculate pseudoparaphyses, anastomosing at the apex, embedded in a hyaline gelatinous matrix. Asci 88–153 × 10–17 μm (\({\overline{\text{x}}}\) = 117.8 × 13.6 μm, n = 20), 8-spored, bitunicate, cylindric-clavate, with furcate or foot-like pedicel, apically rounded, with well-developed ocular chamber. Ascospores 34–47 × 5.7–8 μm (\({\overline{\text{x}}}\) = 41 × 6.8 μm, n = 30), biseriate, yellowish brown, narrowly fusiform, 1-septate, up to 5-septate with age, slightly constricted at the central septum, rough-walled with minute striations, with paler end cells.
Cultural characteristics: Ascospores germinating on WA medium and germ tubes produced from both ends within 12 h. Colonies on PDA medium grow slowly, reaching about 25 mm diam. after 2 months at 25 °C in dark, circular, with dense, dark green mycelium on the surface; in reverse dark green to black with entire margin.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying wood submerged in Suoluo River, 17 October 2018, J. Yang, GDT2-2 (GZAAS 22-2202), living cultures MFLUCC 19-0288 and GZCC 20-0469; CHINA, Guizhou Province, Chishui City, 28.417° N, 106° E, on submerged decaying wood in a freshwater stream, July 2019, L.L. Liu, CS3-9 (GZAAS 20-0373), living culture GZCC 19-0478.
Notes: Our collections match with the holotype of Pseudoastrosphaeriella bambusae in the morphology and dimensions of ascomata, asci and ascospores (Phookamsak et al. 2015b; Dong et al. 2020b). The phylogenetic analysis supports the identification of our collections as P. bambusae (Fig. 1). In this study, we report a new geographical record of this species in China.
Testudinaceae Arx
Notes: Von Arx (1971) introduced Testudinaceae with Testudina as the type genus. The taxonomic history of the members in Testudinaceae was briefly introduced in Calabon et al. (2020a) and Hongsanan et al. (2020a). Currently nine sexual genera are accepted in the family with molecular data available for seven. Recently, the asexual genus Mycoenterolobium formed the basal branch of Testudinaceae based on the molecular analysis (Calabon et al. 2020a). In this study, we treat Mycoenterolobium as a member of Testudinaceae. Members of Testudinaceae are mostly saprobic on dead wood or leaves and some are parasitic on fungi or pathogenic on humans or isolated from soil. They have been reported from terrestrial and aquatic habitats (Bizzozero 1885; von Arx 1971; Hawksworth 1979; Kohlmeyer and Volkmann-Kohlmeyer 1990; Leal et al. 2009; Li et al. 2016a; Wanasinghe et al. 2017; Dayarathne et al. 2020; Hongsanan et al. 2020a).
Mycoenterolobium Goos
Notes: Mycoenterolobium comprises four species characterized by micronematous conidiophores, gregarious, massive, flattened, fan-shaped, dark brown to black conidia (Goos 1970; Index Fungorum 2022). Mycoenterolobium resembles Cancellidium in the large, flattened conidia with rows of cells. However, they are distinguished by the arrangement of conidial rows of cells that radiate from the basal cell in Mycoenterolobium while parallel rows compact at the base in Cancellidium. The scanning electron micrograph study of M. platysporum showed its conidial development/ontogeny differed from Cancellidium (Nakagiri 1993). In addition, conidia of Cancellidium possess internal monilioid cells and the septal pores lacking in Mycoenterolobium (Ho and Hyde 2004; Zelski et al. 2014). Molecular DNA data revealed their phylogenetic placement in Dothideomycetes and Sordariomycetes, respectively (Calabon et al. 2020a; Hyde et al. 2021a).
Mycoenterolobium macrosporum J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF559603; Facesoffungi number: FoF12793; Fig. 36
Etymology: referring to the large conidia
Holotype: MFLU 22-0058
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates effuse, black, glistening, scattered or often aggregated compactly in groups. Mycelium mostly immersed. Conidiophores micronematous. Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical to irregularly shaped. Conidia acrogenous, solitary, fan-shaped or irregular shaped, dark olivaceous green to dark brown, dictyoseptate, formed from the tips of multiple hyphal strands, strongly flattened, composed of many radiating rows of cells arising from the point of attachment, 93–200 µm long, 90–232 µm wide, smooth-walled. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on PDA medium within 24 h and germ tubes produced from each tip of conidial rows. Colonies on MEA medium reaching 5–10 mm diam. in a week at 25 °C, in natural light, circular, with velvety, dense, brown mycelium on the surface; in reverse dark brown to black with entire margin.
Material examined: THAILAND, Phang Nga Province, Bann Tom Thong Khang, on decaying wood submerged in a freshwater stream, 17 December 2015, J. Yang, site7-2-2 (MFLU 22-0058, holotype; HKAS 112179, isotype), ex-type cultures MFLUCC 16-0856.
Notes: Mycoenterolobium macrosporum well matches the generic concept of Mycoenterolobium. Among the four species in the genus, molecular DNA data is only available for a recently introduced species M. aquadictyosporium (Calabon et al. 2020a). In our phylogenetic analysis (Fig. 1), M. macrosporum was positioned as a sister taxon to M. aquadictyosporium with strong support. Comparisons of the LSU, SSU, and ITS sequences of M. macrosporum and M. aquadictyosporium revealed 99.51% (819/823 bp), 99.9% (1010/1011 bp) and 96.35% (793/823 bp, nine gaps) similarity, respectively. They are separate taxa based on the sequence data recommended by Jeewon and Hyde (2016). Mycoenterolobium macrosporum can be distinguished from other species in the genus by conspicuous larger conidia (93–200 × 90–232 µm, this study) that are 45–92 × 43–104 µm in M. aquadictyosporium (Calabon et al. 2020a), 25–100 × 20–60 µm in M. borivaliense (Dubey and Pandey 2020), 23.5–37.5 × 24.5– 45.5 µm in M. flabelliforme (Karandikar et al. 2015) and 110–130 × 75–80 µm in the type species M. platysporum (Goos 1970). However, the conidial dimension of M. macrosporum is similar to M. platysporum var. magnum (85–153 × 95–246 µm) (Dubey and Pandey 2020). We prefer to recognize them as separate species until further sequence data becomes available.
Tetraplosphaeriaceae Kaz. Tanaka & K. Hiray.
Notes: Tetraplosphaeriaceae was established by Tanaka et al. (2009) to accommodate five new genera isolated from bamboo, Polyplosphaeria, Pseudotetraploa, Quadricrura, Tetraplosphaeria and Triplosphaeria. Tetraplosphaeria was designated as the type genus and synonymized under Tetropla due to nomenclatural priority (Hyde et al. 2013). Delgado et al. (2017) added Ernakulamia to Tetraplosphaeriaceae based on the molecular analysis using ITS and β-tubulin gene regions. Ariyawansa et al. (2015a) referred Shrungabeeja to Tetraplosphaeriaceae based on the phylogenetic placement of S. longiappendiculata. Pem et al. (2019) accepted a sexual genus Byssolophis in Tetraplosphaeriaceae. Li et al. (2021b) introduced Aquatisphaeria to the family. The family comprises nine genera and an unclassified species Polyplosphaeria thailandica (Hongsanan et al. 2020a; Wijayawardene et al. 2020). Sexual morphs in Tetraplosphaeriaceae are massarina-like characterized by hyaline, 1–3-septate ascospores surrounded by a sheath, and asexual morphs are characterized by conidia with setose appendages (Tanaka et al. 2009; Hyde et al. 2013; Tibpromma et al. 2018).
Shrungabeeja V.G. Rao & K.A. Reddy
Notes: Shrungabeeja was established by Rao and Reddy (1981) with S. vadirajensis as the type species. The genus is characterized by macronematous, mononematous conidiophores, monoblastic conidiogenous cells, aseptate, subglobose or turbinate conidia with filiform or horn-like appendages. Sexual morphs of Shrungabeeja are unknown. Zhang et al. (2009b) described Shrungabeeja begonia and S. melicopes from China based on morphological characteristics. Ariyawansa et al. (2015a) provided sequence data for S. longiappendiculata and assigned Shrungabeeja in Tetraplosphaeriaceae. Recently, the recollection of the type species and a new species S. aquatica was reported from a freshwater habitat (Dong et al. 2020b). Shrungabeeja is similar to Aquatisphaeria in having macronematous conidiophores and subglobose or turbinate conidia with 3–4 appendages. However, Aquatisphaeria differs by septate conidia.
Shrungabeeja fluviatilis J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559604; Facesoffungi number: FoF12794; Fig. 37
Etymology: referring to the collecting site of a stream.
Holotype: HKAS 112611
Saprobic on submerged twig in freshwater habitats. Asexual morph: Colonies on substrate superficial, effuse, hairy, dark brown, scattered or in small groups. Mycelium mostly immersed, composed of branched, septate, pale brown to brown hyphae. Conidiophores macronematous, mononematous, solitary or in small groups, erect, straight or slightly flexuous, cylindrical, smooth-walled, septate, dark brown, slightly paler towards the apex, 97–260 × 5–10 µm (\({\overline{\text{x}}}\) = 166 × 7.6 µm, n = 20), 3–4.5 µm wide at the apex. Conidiogenous cells integrated, monoblastic, terminal, determinate, cylindrical, brown, truncate at the apex. Conidia acrogenous, solitary, subglobose to napiform, with a tubular base, brown, 30–53 × 21–43 µm (\({\overline{\text{x}}}\) = 40 × 35 µm, n = 30), with four filiform, brown setulae of 24–242 µm long and 1.5–3 µm wide. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Germ tubes produced from the arm tips. Colonies on PDA medium reaching 10 mm diam. after 3 weeks at 25 °C in dark, circular, with velvety, grayish green aerial mycelium on the surface; in reverse black with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on submerged decaying twig in a freshwater stream, 11 July 2019, J. Yang, CS28-1 (HKAS 112611, holotype; HKAS 125924, isotype), ex-type culture GZCC 20-0505; CHINA, Guizhou Province, Chishui City, on submerged decaying twig in a freshwater stream, 16 July 2019, L.L. Liu CS1-2–3 (GZAAS 20-0406, paratype), ex-paratype culture GZCC 19-0511.
Notes: The phylogenetic analysis showed that Shrungabeeja fluviatilis nested within Shrungabeeja and close to S. longiappendiculata (Fig. 1). Shrungabeeja fluviatilis resembles S. aquatica in conidial size and shape, and the number of appendages. However, the latter has obvious percurrent proliferations of conidiogenous cells, which are not observed in Shrungabeeja fluviatilis. Besides, the new taxon has longer appendages than S. aquatica (24–242 µm vs. 12–135 µm). Shrungabeeja longiappendiculata has much longer appendages (up to 480 µm), which makes it different from other Shrungabeeja species (Ariyawansa et al. 2015a). Shrungabeeja fluviatilis differs from S. aquatica and S. longiappendiculata by 14 bp (320/334 bp, one gap) and 24 bp (742/766 bp, 11 gaps) of the ITS sequences, respectively.
Torulaceae Corda
Notes: Torulaceae comprises six genera Dendryphion, Neotorula, Rostriconidium, Rutola, Sporidesmioides and Torula (Crous et al. 2015b, 2020a; Li et al. 2016b; Su et al. 2016a, 2018). Members of the family are only known in asexual morphs (Hongsanan et al. 2020a; Li et al. 2020).
Rostriconidium Z.L. Luo, K.D. Hyde & H.Y. Su
Notes: The genus is characterized by macronematous conidiophores, monotretic to mostly polytretic, cicatrized conidiogenous cells and rostrate conidia with a basal darkened scar (Su et al. 2018; Tibpromma et al. 2018; Shen et al. 2021). Rostriconidium aquaticum, R. cangshanense and R. pandanicola are accepted in the genus. They were collected on decaying submerged wood or Pandanus leaves.
Rostriconidium aquaticum Z.L. Luo, K.D. Hyde & H.Y. Su, Mycol Prog 17(5): 536 (2018)
Index Fungorum number: IF823173; Facesoffungi number: FoF03765; Fig. 38
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates effuse, hairy, dark brown, glistening. Mycelium partly immersed and partly superficial. Conidiophores macronematous, monomematous, solitary or caespitose, cylindrical, straight or slightly flexuous, brown to dark brown, slightly swollen at the base, smooth-walled, 5–8-septate, 175–340 × 9–16 μm (\({\overline{\text{x}}}\) = 252 × 13 μm, n = 20). Conidiogenous cells monotretic or polytretic, integrated, terminal or intercalary, cylindrical, brown, rounded at the apex. Conidia acrogenous or acropleurogenous, solitary, rostrate, straight or slightly curved, dark brown, apical cells and the basal cell pale brown, with a darkened scar at the truncate based, 6–8-septate, slightly constricted at some septa, 93–120 × 18–30 μm (\({\overline{\text{x}}}\) = 110 × 25.5 μm, n = 20), smooth-walled, with a mucilaginous sheath at the apex. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Germ tubes produced from the apex. Colonies on PDA medium reaching 10 mm diam. after 1 week at 25 °C in natural light, circular, gray at first becoming dark, with dense aerial mycelium in the middle and sparse mycelium at edge; in reverse dark brown to black with entire margin.
Material examined: CHINA, Guizhou Province, Guiyang City, Baihua Lake, 26.655° N, 106.537° E, on decaying twigs submerged in the lake, 18 May 2018, L.L. Liu, 18B-35 (GZAAS 20-0319), living culture GZCC 19-0424.
Notes: Rostriconidium aquaticum and R. pandanicola are similar in morphology but can be distinguished by the dimensions of conidiophores and conidia and molecular DNA data. Our collection matches with the generic concept of Rostriconidium. It has shorter conidiophores than R. aquaticum and R. pandanicola (178–343 μm vs. 370–590 μm and 360–485 μm). Conidia of our collection [93–120 μm (\({\overline{\text{x}}}\) = 110 μm)] are shorter than that of R. aquaticum [134–180 μm (\({\overline{\text{x}}}\) = 157 μm)] but longer than R. pandanicola [55–110 μm (\({\overline{\text{x}}}\) = 69.5 μm)]. In the phylogenetic tree (Fig. 1), our collection formed a sister clade to the ex-type strain of R. aquaticum. The LSU, ITS, and TEF1α sequences of our collection and the type material of R. aquaticum exhibit 100%, 99.81% and 99.88% similarity, respectively. We therefore recognize our collection as R. aquaticum based on the molecular evidence.
Tubeufiales Boonmee & K.D. Hyde
Notes: For a brief introduction of the order, see Hongsanan et al. (2020b).
Tubeufiaceae M.E. Barr
Notes: For a brief illustration and description of the family, see Hongsanan et al. (2020b).
Helicoarctatus Y.Z. Lu, J.C. Kang & K.D. Hyde
Notes: Helicoarctatus was introduced by Lu et al. (2018b) with H. aquaticus as the type species. The genus contains two species on decaying wood from freshwater habitats in Thailand (Lu et al. 2018b; Yuan et al. 2020). Helicoarctatus is similar to Helicosporium in the morphology of conidiophores, conidiogenous cells and conidia, but they are distinguished by molecular DNA data.
Helicoarctatus aquaticus Y.Z. Lu, J.C. Kang & K.D. Hyde, Fungal Divers 92: 166 (2018)
Index Fungorum: IF554831; Facesoffungi number: FoF04708; Fig. 40
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates superficial, effuse, gregarious, dark brown, with glistening conidia. Mycelium partly immersed, partly superficial, composed of pale brown to brown, septate, branched hyphae. Conidiophores macronematous, mononematous, setiform, cylindrical, unbranched, septate, dark brown to reddish brown, paler and rounded at the apex, smooth-walled, 155–415 µm long, 4–8 µm wide at the middle part, arising laterally from lower portion of the conidiophores with orange to reddish brown, intercalary conidia-like masses on the conidiophore. Conidiogenous cells mono- to polyblastic, determinate, terminal or intercalary, denticulate, arising laterally from lower portion of the conidiophores, hyaline, lageniform, smooth-walled, or fertile at the apex of conidiophores, 3.5–11 × 2–4 µm. Conidia solitary, acropleurogenous, helicoid, rounded at the tip, 30–59 μm diam. and conidial filaments 2.5–5 μm wide, 245–335 μm long, tightly coiled 3½–4½ times, becoming loosely coiled in water, indistinctly multi-septate, guttulate, hyaline, smooth-walled. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on PDA medium within 24 h. Colonies on PDA medium slow growing, reaching 10–15 mm diam. in 1 month at 25 °C, in natural light, circular, with dense, dark brown mycelium on the umbonate surface; in reverse dark brown or black with irregular margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT7-3 (MFLU 22-0065 and HKAS 112150), living cultures MFLUCC 17-2054 and GZCC 20-0379.
Notes: In the phylogenetic analysis, our collection (MFLUCC 17-2054) was positioned as sister to H. aquaticus (MFLUCC 17-1996) with strong support (100% MLBS/1.0 PP) (Fig. 39). However, only LSU sequence data is available for both strains with 100% similarity. Our collection matches with the type material of H. aquaticus in the morphology except the conidia are longer and some become loosely coiled in water. We recognize our collection as H. aquaticus but sequence data of ITS, TEF1α, and RPB2 genes are expected to confirm its taxonomy. In this study, we described an additional character of the red spots on the conidiophores which was ignored in the original diagnosis of H. aquaticus (Lu et al. 2018b).
Maximum likelihood majority rule consensus tree for Tubeufiales using LSU, ITS, TEF1α, and RPB2 sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated above branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Botryosphaeria agaves (MFLUCC 10–0051) and Botryosphaeria dothidea (CBS 115476). The new collections are in bold and new taxa in blue. Ex-type strains are indicated with T after the strain number. Branches with 100% ML BS and 1.0 PP are thickened
Helicoarctatus aquaticus (MFLU 22-0065) a Colony on wood. b, c Conidiophores. d, e Fertile apex of conidiophores. f–h Conidiogenous cells. i–k Conidia-like masses. l–o Conidia. p Germinated conidium. q, r Culture, q from above, r from below. Scale bars: a = 200 µm, b, c = 100 µm, p = 40 µm, g, i, j, l = 30 µm, d–f, h, m–o = 20 µm, k = 10 µm
Neohelicomyces Z.L. Luo, Bhat & K.D. Hyde
Notes: Luo et al. (2017) established Neohelicomyces typified by N. aquaticus. The genus is characterized by macronematous, mononematous, elongated, hyaline to pale brown, sparsely branched, septate conidiophores, mono- to poly-blastic, integrated conidiogenous cells with lateral minute denticles and acropleurogenous, helicoid conidia (Luo et al. 2017; Lu et al. 2018b). Eleven asexual taxa are accepted in the genus (Index Fungorum 2022). They are saprobic on decaying wood, culms, or leaves, mostly from freshwater habitats and several are terrestrial.
Neohelicomyces denticulatus L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
Index Fungorum number: IF559605; Facesoffungi number: FoF12795; Fig. 41
Etymology: referring to the denticulate conidiogenous cells.
Holotype: GZAAS 20-0339
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, gregarious, pale yellow, with glistening conidial masses. Mycelium mostly superficial and partly immersed, composed of branched, septate, thin-walled, smooth, subhyaline hyphae. Conidiophores macronematous, mononematous, cylindrical, pale brown, paler towards the apex, straight or flexuous, branched, septate, tapering distally, 111–236 × 3–6.5 μm (\({\overline{\text{x}}}\) = 166 × 4.5 μm, n = 20). Conidiogenous cells polyblastic, integrated, terminal or intercalary, determinate, sympodial, denticulate; denticles tiny, cylindrical. Conidia acropleurogenous, solitary, hyaline, helicoid, multi-septate, rounded at tip, guttulate, tightly coiled 2½–3½ times, becoming loosely coiled in water, 16–22 μm diam., conidial filaments 1.5–2.5 μm (\({\overline{\text{x}}}\) = 2 μm, n = 20) wide, 83–121 μm (\({\overline{\text{x}}}\) = 105.5 μm, n = 20) long, smooth-walled. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium and germ tubes produced within 12 h. Colonies growing on PDA medium, reaching about 10–15 mm in 3 weeks at 25 ℃ in dark, circular, with velvety brown mycelium on the umbonate surface; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Guiyang City, Baihua Lake, 26.655° N, 106.537° E, on decaying wood submerged in the lake, 18 April 2018, L.L. Liu, 18B-30 (GZAAS 20-0339, holotype), ex-type culture GZCC 19-0444.
Notes: Among Neohelicomyces species, N. denticulatus is similar to N. pandanicola in morphology and dimensions of conidiophores and conidia, except for more denticles (up to 11 vs. 1–3) on conidiogenous cells (Tibpromma et al. 2018). In our phylogenetic tree (Fig. 39), Neohelicomyces denticulatus was positioned as sister to N. pandanicola (KUMCC 16-0143) with weak support. Comparisons of LSU and ITS sequences of N. denticulatus and N. pandanicola revealed 99.87% (795/796 bp) and 96.45% (462/479 bp) sequence similarity, respectively.
Neohelicosporium Y.Z. Lu, J.C. Kang & K.D. Hyde
Notes: Neohelicosporium was introduced to accommodate helicosporous taxa that are characterized by branched or unbranched conidiophores arising from creeping hyphae, mono- to polyblastic, integrated, sympodial conidiogenous cells with denticles and acrogenous and/or acropleurogenous conidia and formed a distinct clade to other genera in Tubeufiaceae (Lu et al. 2018a). So far, 25 species are accepted in the genus with some transferred from Helicoma, Helicomyces, Helicosporium and Tubeufia (Chethana et al. 2021; Index Fungorum 2022). Sexual-asexual connections were described for Neohelicosporium ellipsoideum, N. fusisporum and N. ovoideum through cultural studies or molecular evidence (Jayasiri et al. 2017; Lu et al. 2018b).
Neohelicosporium fluviatile J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF559606; Facesoffungi number: FoF12796; Fig. 42
Neohelicosporium fluviatile (MFLU 15-1141, holotype) a Colony on wood. b, c Conidiophores with conidia. d–f Conidiogenous cells. g–l Conidia. m Germinated conidium on PDA medium. n, o Culture on MEA medium, n from above, o from below. Scale bars: a = 200 μm, b, e, g–m = 30 μm, c, f = 20 μm, d = 15 μm
Etymology: referring to the habitat of a stream.
Holotype: MFLU 15-1141
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates superficial, effuse, gregarious, white, glistening. Mycelium partly immersed, partly superficial, composed of pale brown to hyaline, septate, branched hyphae. Conidiophores macronematous, mononematous, cylindrical, branched, septate, up to 85 µm long, 4–6.5 µm wide, pale brown to hyaline, smooth-walled. Conidiogenous cells mono- to polyblastic, integrated, terminal or intercalary, cylindrical, pale brown to hyaline, denticulate, smooth-walled. Conidia holoblastic, solitary, acropleurogenous, helicoid, rounded at both ends, 28–38 μm diam. when tightly compact and conidial filaments 215–430 × 3–7.5 µm (\({\overline{\text{x}}}\) = 330 × 5.6 µm, n = 25), tightly coiled 3½–4½ times, becoming loosely coiled in water, indistinctly multi-septate, guttulate, hyaline, becoming pale brown with age, smooth-walled, sometimes with pale brown basal cells. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium and germ tubes produced within 12 h. Colonies growing on MEA medium, reaching 5–10 mm in 2 weeks at 25 °C, in natural light, circular, with dense, velvety, dark brown mycelium on the surface; in reverse dark brown to black with filiform margin.
Material examined: THAILAND, Prachuap Khiri Khan Province, Hua Hin, near Pala-U Waterfall, on submerged decaying wood in a freshwater stream, 25 December 2014, J. van Strien, site4-1-3 (MFLU 15-1141, holotype; HKAS 112583, isotype), ex-type culture MFLUCC 15-0606.
Notes: Neohelicosporium fluviatile resembles N. krabiense in having pale brown conidiophores, mono- to polyblastic, integrated, terminal or intercalary conidiogenous cells with tooth-like paler denticles and conidia loosely coiled in water (Lu et al. 2018b). However, conidial filaments of Neohelicosporium fluviatile are longer and broader than that in N. krabiense (215–430 × 3–7.5 µm vs. 230–320 × 3–4 µm). Conidiophores of N. fluviatile are often sparsely branched while that are unbranched in N. krabiense. The phylogenetic analysis of combined LSU, ITS, TEF1α, and RPB2 sequences indicated that N. fluviatile (MFLUCC 15-0606) placed as a sister taxon to N. krabiense (MFLUCC 16-0224) (Fig. 39). The LSU, TEF1α, and RPB2 sequences of N. fluviatile and N. krabiense exhibit 99.34% (1212/1220 bp), 98.91% (813/822 bp) and 98.28% (1027/1045 bp) sequence similarity, respectively. However, an ITS sequence is not available for N. fluviatile. Given the molecular evidence and their morphological difference, we therefore treat them as separate species. Neohelicosporium fluviatile clustered close to Neohelicosporium sp. (HKUCC 10235) which was initially recognized as Helicomyces macrofilamentosus but lacked morphological data. Lu et al. (2018b) considered this strain misidentified and treated it as a member of Neohelicosporium based on the molecular phylogeny.
Pleurohelicosporium Y.Z. Lu, J.C. Kang & K.D. Hyde.
Notes: Lu et al. (2018b) established the monotypic genus Pleurohelicosporium based on the separate systematic placement from other genera in Tubeufiaceae. It can be distinguished from Neohelicosporium by pleurogenous conidia which are acrogenous or acropleurogenous in the latter (Lu et al. 2018b).
Pleurohelicosporium hyalinum J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF900058; Facesoffungi number: FoF12797; Fig. 43
Etymology: referring to the hyaline conidia.
Holotype: MFLU 22-0231
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates superficial, effuse, gregarious, white, with glistening conidial masses. Mycelium composed of partly immersed, partly superficial, hyaline, septate, branched hyphae. Conidiophores macronematous, mononematous, cylindrical, branched, septate, straight or flexuous, 20–150 μm long, 2.5–5 μm wide, hyaline, smooth-walled. Conidiogenous cells mono- to polyblastic, integrated, terminal or intercalary, cylindrical, denticulate, hyaline, smooth-walled. Conidia solitary, acropleurogenous, helicoid, rounded at both ends, 15–20 μm diam. when tightly coiled and conidial filaments 2–3.5 μm wide, (140–)190–230 μm long, tightly coiled 2½–3½ times, becoming loosely coiled in water, indistinctly multi-septate, guttulate, hyaline, smooth-walled. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium within 24 h. Colonies on PDA medium reaching 10–15 mm diam. in 1 month at 25 °C in dark, circular, with dense, dark brown mycelium on the umbonate surface; in reverse dark brown to black with filiform margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou scenic spot, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS6-3 (MFLU 22-0231, holotype), ex-type culture GZCC 20-0489.
Notes: In the phylogenetic analysis (Fig. 39), Pleurohelicosporium hyalinum (GZCC 20-0489) was placed as sister to P. parvisporum (MFLUCC 17-1982) with good support (96% MLBS/1.0 PP). Pleurohelicosporium hyalinum is characterized by hyaline conidiophores, polyblastic, terminal and intercalary conidiogenous cells and acropleurogenous conidia while P. parvisporum produces pale brown conidiophores, monoblastic, intercalary conidiogenous cells and pleurogenous conidia. Sequence similarity of LSU, ITS, TEF1α, and RPB2 gene regions between both species are 95.62% (1223/1279 bp), 90.33% (486/538 bp), 96.82% (883/912 bp) and 93.87% (980/1044 bp), respectively. Given the different morphology and low sequence similarity in the studied loci, we recognize P. hyalinum as a new species.
Dothideomycetes genera incertae sedis
Diplocladiella G. Arnaud ex M.B. Ellis
Notes: Diplocladiella was established by Arnaud (1954) with D. scalaroides as the type species. The taxon was validated by Ellis (1976) with a Latin diagnosis. Diplocladiella comprises eight species characterized by macronematous, mononematous, pale to mid brown conidiophores, polyblastic, sympodial, geniculate conidiogenous cells with cicatrized, truncate conidiogenous loci and broadly Y-shaped, conidia, subhyaline when young, becoming pale olivaceous green, pale brown to brown, sometimes with pale to hyaline, filiform appendages at the arms’ tip. Sexual morphs are still unknown in the genus, and molecular DNA data is also unavailable.
Diplocladiella scalaroides G. Arnaud ex M.B. Ellis, More Dematiaceous Hyphomycetes (Kew): 229 (1976)
Index Fungorum number: IF296790; Facesoffungi number: FoF12798; Fig. 44
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, subhyaline to pale brown, velvety, glistening, with masses of conidia on conidiophores. Mycelium mostly immersed, consisting of branched, septate, smooth-walled, subhyaline to pale brown hyphae. Conidiophores macronematous, mononematous, smooth-walled, septate, erect, straight or slightly curved, flexuous on conidiogenesis part, solitary or fasiculate, cylindrical, hyaline to pale brown, 19–48 × 2.5–4 μm. Conidiogenous cells polyblastic, integrated, terminal, determinate, hyaline or subhyaline to pale brown, geniculate, with sympodial proliferations. Conidia acropleurogenous, solitary, Y-shaped, with two horned arms, a middle pentagonal cell and trapezoidal basal cell from front view, septate, smooth-walled, hyaline when young, becoming pale olivaceous to pale brown, the two tip cells hyaline to subhyaline, sometimes extending, the basal cell hyaline to subhyaline or pale brown, axis 10–12.5 μm long (\({\overline{\text{x}}}\) = 10.8 µm, n = 30). (Fig. 44, m1 yellow line), pentagonal cells 5–7 μm wide (\({\overline{\text{x}}}\) = 5.9 µm, n = 30) (Fig. 44, m2 green line), arms 8–16 × 4.5–7 μm (\({\overline{\text{x}}}\) = 11.8 × 5.6 µm, n = 60) (Fig. 44, m4 blue line and m3 orange line), 17–26(–30) µm wide between two horn tips. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium within 24 h and germ tubes produced from the arm tips. Colonies on PDA medium slow growing, reaching around 10 mm diam. after 2 months at 25 °C in dark, circular, aerial mycelium dense, velvety, greyish white in the middle, dark olivaceous at edge; in reverse dark with entire or filiform margin.
Material examined: CHINA, Guizhou Province, Bijie City, Weining, Caohai national nature reserve, 26.817° N, 104.217° E, on decaying submerged aquatic plants in Caohai Lake, L.L. Liu, October 2018, 18C-46 (GZAAS 20-0458, reference specimen designated here), living culture GZCC19-0563.
Notes: Diplocladiella scalaroides, the generic type, is a common saprobe worldwide. Sati et al. (2008) reported the fungus as a root endophyte. It has been discovered from various terrestrial and aquatic elements on dead leaves, decaying wood, foam and particularly from the air in a greenhouse, rainwater on trees, water-filled tree holes, and even honey (Ingold 1975; Matsushima 1980; Kirk 1982; Hyde and Jones 1989; Cooper 2005; Gönczöl and Révay 2003, 2004; Magyar et al. 2005, 2011b). Our collection is identified as Diplocladiella scalaroides based on morphological characters. The conidiophores and conidial dimensions of our collection are comparable to that in Ellis (1976) and arms are shorter than that in Matsushima (1975). We designate our collection as a reference specimen for Diplocladiella scalaroides with sequence data generated of LSU, ITS, and SSU gene regions. In the phylogenetic analysis (Fig. 1), D. scalaroides clustered with Oncopodiella trigonella as genera incertae sedis within Dothideomycetes.
Oncopodiella G. Arnaud ex Rifai
Notes: Arnaud (1954) introduced Oncopodiella with a single species O. tetraedrica. However, they were not validly published and lacked a Latin diagnosis. Rifai (1965) formally published the generic name and combined O. tetraedrica with Sporidesmium trigonellum as the type species O. trigonella based on the same morphology. Oncopodiella possesses macronematous, mononematous, subhyaline to pale brown conidiophores, polyblastic, sympodial conidiogenous cells with cylindrical denticles and dematiaceous, muriform, corniculate conidia in various shapes (trigonous, turbinate, subglobose, ellipsoidal, obovoid, pyriform and fusiform or irregular) with hyaline or subhyaline horned, protruding cells (Rifai 1965; Ellis 1971; Zhao and Zhang 2005b; Magyar and Révay 2009). So far, 13 species are accepted in the genus with no known sexual morphs. Hernández-Restrepo et al. (2017) designated an epitype for O. trigonella, the generic type, and provided the sequence data which showed that O. trigonella (FMR 10788 = CBS 126413) is a member of Dothideomycetes but unrelated to other taxa and represents a potentially new fungal lineage.
Oncopodiella trigonella (Sacc.) Rifai, Persoonia 3(4): 409 (1965)
Index Fungorum number: IF335397; Facesoffungi number: FoF12349; Fig. 45
Basionym: Sporidesmium trigonellum Sacc., Michelia 2(8): 641 (1882)
Saprobic on submerged decaying wood in a freshwater stream. Asexual morph: Colonies dark brown, punctiform or granular. Conidiophores macronematous, mononematous, smooth-walled, subhyaline to pale brown, or reduced to conidiogenous cells. Conidiogenous cells monoblastic. Conidia solitary, subglobose, smooth-walled, muriform, brown, corniculate, 12–19 × 12–16 μm (\({\overline{\text{x}}}\) = 16 × 14 µm, n = 30), with 2–4, mostly 3, hyaline to pale brown, horn-like protruding cells. Sexual morph: Undetermined.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying wood submerged in Suoluo River, 17 October 2018, J. Yang, GDT3-1 (HKAS 112637).
Notes: Attempts to preserve the living culture were unsuccessful since few conidia germinated, with no growth after reaching 1–2 mm diam.
This collection fits well with the holotype of O. trigonella in morphology and conidial dimension but has smaller conidia than the epitype (17–23.5 × 10.5–16.5 μm) (Rifai 1965; Hernández-Restrepo et al. 2017). The phylogenetic analysis showed our collection (HKAS 112637) clustered together with the ex-epitype (FMR 10788 = CBS 126413) of O. trigonella and they were sister to Diplocladiella scalaroides (GZCC 19-0563) (Fig. 1). These three strains formed a separate clade with their phylogenetic placement remaining unresolved in Dothideomycetes.
Oncopodiella trigonella is the most common species of the genus. It was reported mainly from Europe including Belgium, Great Britain, France, Hungary, Italy, Lithuania and Spain, and also from Asia in Iran and China. This species frequently occurs on bark, dead branches, leaves and plant debris and is encountered in rainwater, honey and water-filled tree holes and is reported as air spores in an Italian vineyard (Rifai 1965; Kirk 1983; Gönczöl and Révay 2003, 2004; Zhao and Zhang 2005b; Gharizadeh et al. 2007; Magyar and Révay 2009; Magyar et al. 2009, 2016; Rambelli et al. 2010; Kutorga et al. 2013; Hernández-Restrepo et al. 2017). This study first reports Oncopodiella trigonella from submerged decaying wood in freshwater habitats.
Eurotiomycetes O.E. Erikss. & Winka
Chaetothyriales M.E. Barr
Notes: Chaetothyriales belongs to Chaetothyriomycetidae in Eurotiomycetes and comprises ten families (Wijayawardene et al. 2022). Species in the order are mainly epiphytes that are often adpressed to the surface of leaves and stems and get nutrients from sugary exudates (Tian et al. 2021). Others can be saprobes or pathogens reported worldwide (Barr 1987; Untereiner and Naveau 1999; Untereiner 2000; Réblová et al. 2013; de Hoog 2014; Hyde et al. 2019; Tian et al. 2021). Some species can be found in extreme and adverse conditions, such as on rock surfaces in hot, arid climates and in toxic niches with hydrocarbons and heavy metals (de Hoog 2014).
Herpotrichiellaceae Munk
Notes: Munk (1953) introduced the family Herpotrichiellaceae, which is currently placed in Chaetothyriales with evidence from phylogenetic analyses (Liu et al. 2015b; Tian et al. 2016; Dong et al. 2018; Wang et al. 2019). The sexual morphs of this family are characterized by ascomata with typical setae and ostioles, bitunicate, saccate to ovoid asci with a thickened apex and pale grey to brown ascospores which may be didymosporous, phragmosporous or dictyosporous (Munk 1953). The asexual morphs are various dematiaceous hyphomycetes including Exophiala, Philaphora, cladophilaphora-like species, Fonsecaea, Capronia (Gueidan et al. 2014), and undetermined pyricularia-like taxa (Gueidan et al. 2014; Klaubauf et al. 2014; Liu et al. 2015b; Tian et al. 2016; Dong et al. 2018). Presently, 17 genera are accepted in Herpotrichiellaceae (Wijayawardene et al. 2022).
Thysanorea Arzanlou, W. Gams & Crous
Notes: Arzanlou et al. (2007) established the genus Thysanorea based on T. papuana (basionym: Periconiella papuana), which has apically branched conidiophores. In the phylogenetic studies, Thysanorea nested within Minimelanolocus clade in Herpotrichiellaceae (Dong et al. 2018; Wang et al. 2019; Hernández-Restrepo et al. 2020; Wan et al. 2021). However, the molecular data is unavailable for the type species of Minimelanolocus. The generic type Minimelanolocus navicularis is characterized by macronematous, mononematous, terminal conidiophores, polyblastic conidiogenous cells and navicular, versicolor conidia with a thickened and darkened middle septum, while species in Minimelanolocus clade produce terminal and intercalary conidiophores and clavate, cylindrical, ellipsoidal, or fusiform, subhyaline to pale brown conidia (Liu et al. 2015b; Costa et al. 2017; Dong et al. 2018; Wang et al. 2019; Wan et al. 2021). Hernández-Restrepo et al. (2020) consequently transferred the Minimelanolocus species that grouped with Thysanorea to the latter genus as they are congeneric in phylogeny. However, the Minimelanolocus species without sequence data remained in the genus. In comparison, this combination was not accepted by Wan et al. (2021) because of the branching pattern of the conidiophores. In this study, we follow Hernández-Restrepo et al. (2020), but the sequence data or epitypification of M. navicularis is needed to confirm the relationship between Minimelanolocus and Thysanorea.
Thysanorea amniculi J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559607; Facesoffungi number: FoF12799; Fig. 47
Etymology: referring to the collecting site of a small stream.
Holotype: HKAS 112582
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates effuse, hairy, scattered or in small groups, brown, with glistening conidial masses at apex. Mycelium partly superficial, partly immersed, composed of septate, smooth, pale brown hyphae. Conidiophores macronematous, mononematous, erect, solitary or in small groups, straight or flexuous, cylindrical, smooth-walled, septate, rarely branched, dark brown, becoming pale brown or subhyaline towards the apex, thick-walled, 190–465 × 4–10 µm (\({\overline{\text{x}}}\) = 330 × 5.7 µm, n = 20). Conidiogenous cells polyblastic, integrated, sympodially proliferating, terminal, later becoming intercalary, cylindrical, wavy on the surface, pale brown or subhyaline, 12–40 × 3–5 µm (\({\overline{\text{x}}}\) = 24.5 × 3.7 µm, n = 20). Conidia acropleurogenous, aggregated in slimy masses, clavate, with a narrow truncate base, aseptate when young, 1–4-septate when mature, smooth, guttulate, hyaline to subhyaline, (13–)15–23 × 2.5–5 µm (\({\overline{\text{x}}}\) = 18.7 × 3.8 µm, n = 50), thin-walled. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 10 mm diam. after 1 week at 25 °C in natural light, circular, gray at first becoming dark, with dense aerial mycelium in the middle and sparse mycelium at margin; dark brown to black in reverse with entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 6 July 2018, J. Yang, SGT69-1 (HKAS 112582, holotype; HKAS 125935, isotype); ex-type cultures MFLUCC 19-0283 and GZCC 20-0465.
Notes: Thysanorea amniculi is similar to T. nonramosa and T. obscura in having mononematous, macronematous, septate conidiophores, polyblastic, integrated, sympodially proliferating conidiogenous cells and clavate conidia with a narrow truncate base (Matsushima 1983; Wang et al. 2019). However, Thysanorea amniculi differs from T. nonramosa in having longer conidiophores (190–465 vs. 270–360), larger conidia (15–23 × 2.5–5 vs. 11–17 × 1.5–4.0 µm) and more conidial septa (1–4 vs. 1–3). Thysanorea amniculi can be easily distinguished from T. obscura by the size of conidiophores (190–465 × 4–10 vs. 123–219 × 4.5–5.5 µm), color (hyaline to subhyaline vs. hyaline to pale brown) and number of conidial septa (1–4 vs. 1–3).
In the phylogenetic analysis using combined ITS, LSU, and SSU sequence data, T. amniculi formed a distinct clade within Thysanorea (Fig. 46). Based on a megablast search of the NCBI GenBank nucleotide sequence database using the ITS sequence of T. amniculi, the highest similarity (97.34%) is with Thysanorea thailandensis (MFLUCC 15-0971) with 16 base pairs differences (586/602 bp, three gaps). However, Thysanorea amniculi is easily distinguished from T. thailandensis in having branched and longer conidiophores (190–465 vs. 130–150 μm) and mostly 3–4-septate and longer conidia (15–23 vs. 9–11 μm), while conidiophores of T. thailandensis are unbranched and conidia are mostly 2-septate (Dong et al. 2018).
Maximum likelihood majority rule consensus tree for Herpotrichiellaceae using ITS, LSU, and SSU sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated above branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Cyphellophora oxyspora (CBS 698.73) and Cyphellophora sessilis (CBS 243.85). The new collections are in bold and new taxa in blue. Ex-type strains are indicated with T after the strain number. Branches with 100% ML BS and 1.0 PP are thickened
Thysanorea nonramosa (X.D. Yu, G.N. Wang & H. Zhang) Hern.-Restr. & Crous, Fungal Syst Evol 6: 18 (2020)
Index Fungorum number: IF833924; Facesoffungi number: FoF12800; Fig. 48
Basionym: Minimelanolocus nonramosus X.D. Yu, G.N. Wang & H. Zhang, Mycol Prog 18: 514 (2019)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates effuse, hairy, scattered or in small groups, brown, with glistening conidial masses at apex. Mycelium partly superficial, partly immersed, composed of septate, smooth, pale brown hyphae. Conidiophores macronematous, mononematous, erect, solitary or in small groups, straight or flexuous, cylindrical, smooth-walled, septate, unbranched, dark brown or mid brown, becoming pale brown or subhyaline towards the apex, thick-walled, 120–230 × 3–8 µm (\({\overline{\text{x}}}\) = 162 × 4.8 µm, n = 30). Conidiogenous cells polyblastic, integrated, sympodially proliferating, terminal becoming intercalary, cylindrical, pale brown or subhyaline, 11.5–34 × 2.5–4 µm, with inconspicuous conidiogenous loci. Conidia acropleurogenous, aggregated in slimy masses, clavate, with a narrow truncate base, hyaline, aseptate when immature, 1–3-septate at maturity, smooth-walled, guttulate, 10–20 × 2.5–5 µm (\({\overline{\text{x}}}\) = 14.5 × 3.5 µm, n = 50), thin-walled, sometimes slightly constricted at septa. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on MEA medium reaching 10 mm diam. after 1 week at 25 °C in natural light, circular, grayish green, with dense mycelium on the ring-like surface; in reverse dark green to black with entire margin.
Material examined: THAILAND, Phang Nga Province, Bann Tom Thong Khang, on decaying wood submerged in a freshwater stream, 17 December 2015, J. Yang, site7-77-1 (MFLU 22-0061 and HKAS 112184); living cultures MFLUCC 16-0877 and GZCC 17-0032.
Notes: Based on a BLAST search on NCBI GenBank, the ITS and LSU sequence of our collection is 99.48% and 99.66% identical to Thysanorea nonramosa (MFLUCC 17-2378). Phylognenetic analysis showed our collection clustered with the ex-type strain of T. nonramosa (MFLUCC 17-2378) (Fig. 46). The morphology of our collection fits well with the holotype of T. nonramosa (Wang et al. 2019). Thus, we recognize our collection as T. nonramosa which is recollected in Thailand.
Veronaea Cif. & Montemart.
Notes: Veronaea was introduced by Cifferi and Montemartini (1958) with V. botryosa as the type species. The genus is characterized by erect, unbranched, brown conidiophores, holoblastic, sympodial conidiogenous cells and 1-septate to a few transversely septate conidia (Ellis 1971; Badali et al. 2013; Dong et al. 2018). Veronaea is similar to Ramichloridium. They share the characteristics as sympodial conidiogenous cells with unpigmented to faintly pigmented conidial scars (Arzanlou et al. 2007). However, Veronaea differs from Ramichloridium in having two-celled conidia with a small, flat pigmented conidial scar (Arzanlou et al. 2007; Soares and Barreto 2008). Phylogenetically, they clustered in different classes, Veronaea in Chaetothyriales (Eurotiomycetes), and Ramichloridium in Capnodiales (Dothideomycetes) (Arzanlou et al. 2007). Thirty epithets are listed under Veronaea with nine of them transferred to Globoramichloridium, Periconiella, Ramichloridium, and Zasmidium (Index Fungorum 2022). Veronaea species were reported as saprobes, mycoparasites and pathogens, and have a worldwide distribution (Soares and Barreto 2008).
Veronaea botryosa Cif. & Montemart., Atti 1st bot Univ Lab crittog, Pavia ser 5 15: 68 (1957)
Index Fungorum number: IF307734; Facesoffungi number: FoF10445; Fig. 49
Synonym: Veronaea constricta Moustafa & Abdul-Wahid, Mycotaxon 38: 167 (1990)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates effuse, hairy, scattered or in small groups, brown. Mycelium mostly immersed. Conidiophores macronematous, mononematous, straight or flexuous, cylindrical, thick-walled, septate, brown, paler towards the apex, (78–)107–180 × 3–5.5 μm (\({\overline{\text{x}}}\) = 136.5 × 4 μm, n = 15). Conidiogenous cells polyblastic, terminal, integrated, sympodial, pale brown to subhyaline. Conidia acropleurogenous, clavate to obovoid, 0–3-septate, slightly constricted at septa, 6–10 × 3–4.5 μm (\({\overline{\text{x}}}\) = 7.8 × 3.5 μm, n = 20), apically rounded, with an obconical basal cell, subhyaline to pale brown, smooth-walled. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium and germ tubes produced from one or both ends within 12 h. Colonies on PDA medium reaching 15–20 mm diam. after 3 weeks at 25 °C in dark, circular, with dense, velvety, grayish brown mycelium in center, grayish green in the middle ring and white at edge; in reverse dark olive-green with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 16 July 2019, Y.Y. Chen, CS1-6 (GZAAS 20-0452); living culture GZCC 19-0557.
Notes: In the phylogenetic analysis inferred from ITS, LSU, and SSU sequence data, our collection grouped with Veronaea botryosa (Fig. 46). Comparisons of the ITS, LSU and SSU gene regions of our collection and the type material of V. botryosa (CBS 254.57) revealed 99.3% (573/577 bp), 99.88% (887/888 bp), and 100% (1019/1019 bp) sequence similarity, respectively. The morphology and dimensions of our collection well match with the holotype and other collections of V. botryosa (Ellis 1971; Arzanlou et al. 2007; Dong et al. 2018). We therefore identify our collection as V. botryosa and report the species from freshwater habitats in China. The strain CBS 572.90 was isolated from the holotype of V. constricta. Based on the high sequence similarity and similar morphology and dimensions between V. constricta and V. botryosa (Moustafa and Abdul-Wahid 1990), we treat V. constricta as a synonym for V. botryosa.
Sclerococcales Réblová, Unter. & W. Gams
Notes: Sclerococcales was placed in the subclass Sclerococcomycetidae. Sclerococcaceae was introduced as the type family by Réblová et al. (2017). Based on phylogenetic analyses, Dactylospora and Sclerococcum appear to represent a monophyletic group (Diederich et al. 2013; Pino-Bodas et al. 2017; Réblová et al. 2017). Thus, Diederich et al. (2018) reinstated Dactylosporaceae to replace Sclerococcaceae and retained Sclerococcum as the type genus with several Dactylospora species transferred to Sclerococcum.
Dactylosporaceae Bellem. & Hafellner
Notes: Dactylosporaceae comprises lignicolous, lichenicolous, and beetle-associated fungi from terrestrial or aquatic habitats. Seven genera were accepted in the family (Wijayawardene et al. 2022).
Gamsomyces Hern.-Restr. & Réblová
Notes: Réblová et al. (2020a) established Gamsomyces to accommodate two asexual fungi with sporodochial or synnematous conidiomata, semi-macronematous or macronematous, fasciculate conidiophores, monoblastic conidiogenous cells and multi-septate, narrowly fusiform conidia with a mucilaginous cap at the apex. Dong et al. (2020a), at the same time, introduced a new genus Pseudobactrodesmium which matches the generic concept of Gamsomyces. Multigene phylogenetic analysis of the combined dataset revealed that both genera represent the same genus (Fig. 50). We therefore recommend Gamsomyces for use following the priority consideration.
Maximum likelihood majority rule consensus tree for Sclerococcales using LSU, ITS, SSU, and RPB2 sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated above branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Trichocoma paradoxa (CBS 788.83) and Eupenicillium javanicum (AFTOL-ID 429). The new collections are in bold and ex-type strains are indicated with T after the strain number. Branches with 100% ML BS and 1.0 PP are thickened
Gamsomyces aquaticus (W. Dong, H. Zhang & K.D. Hyde) J. Yang & K.D. Hyde, comb. nov.
Index Fungorum number: IF559611; Facesoffungi number: FoF12801
Basionym: Pseudobactrodesmium aquaticum W. Dong, H. Zhang & K.D. Hyde, Front Microbiol 11(no. 565): 5 (2020)
Holotype: CHINA, Yunnan Province, Pingbian City, on submerged wood in a stream, 20 September 2017, W. Dong, WF-24A-1 (MFLU 18-1171, holotype), ex-type culture MFLUCC 18-1015.
Notes: The genera Gamsomyces was introduced earlier than Pseudobactrodesmium (Dong et al. 2020a; Réblová et al. 2020a). Morphology and molecular data revealed they represent the same genus (Boonmee et al. 2021; this study). Gamsomyces takes priority and we therefore transfer Pseudobactrodesmium aquaticum to Gamsomyces.
Gamsomyces longisporus (M.B. Ellis) Hern.-Restr. & Réblová, Stud Mycol 95: 449 (2020)
Index Fungorum number: IF834448; Facesoffungi number: FoF12802; Fig. 51
Basionym: Bactrodesmium longisporum M.B. Ellis, More Dematiaceous Hyphomycetes: 68 (1976)
Synonym: Pseudobactrodesmium chiangmaiense X.D. Yu, W. Dong & K.D. Hyde, Front Microbiol 11(no. 565): 5 (2020)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Conidiomata on natural substrate sporodochial, superficial, scattered, dark brown, with glistening conidial masses at apex. Mycelium mostly immersed, composed of septate, smooth-walled, pale brown hyphae. Conidiophores semi-macronematous to macronematous, fasciculate, erect, straight or slightly flexuous, cylindrical, smooth-walled, septate, unbranched or branched, mid brown, often paler towards the apex, tightly compact forming short synnemata, thick-walled, (10–)30–65 × 2–3 µm (\({\overline{\text{x}}}\) = 40 × 2.5 µm, n = 20). Conidiogenous cells monoblastic, integrated, terminal or intercalary, cylindrical or slightly swollen, pale brown to subhyaline. Conidia acrogenous, narrowly fusiform, elongated, straight or slightly curved, truncate at the base, rounded to subulate at the apex, 14–21-septate, smooth-walled, guttulate, pale greenish brown to pale brown, becoming hyaline at the apical cells, 70–112 × 5–7.5 µm (\({\overline{\text{x}}}\) = 87 × 6.5 µm, n = 30), often with a globose mucilaginous cap apically (12–16 µm diam.). Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on PDA medium within 12 h and germ tubes can produce from every cell. Colonies on PDA medium reaching 5–10 mm diam. in a week at 25 °C in dark, circular, with fluffy, dense, dark brown mycelium on the surface with a grayish middle ring; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.917° N, 107.617° E, 1205 m, on decaying wood submerged in a freshwater stream, 5 July 2018, L.L. Liu, 18D-42 (GZAAS 20-0358), living culture GZCC 19-0463; ibid.18D-36-3 (GZAAS20-0357), living culture GZCC 19-0462.
Notes: Gamsomyces longisporus was originally described as Bactrodesmium longisporum with sporodochial conidiomata of the holotype (Ellis 1976). In an Indian specimen of G. longisporus, both synnemata and sporodochia were formed on the natural substrate (Rao and de Hoog 1986; Réblová et al. 2020a). The synnemata and sporodochia were considered as the same conidiomatal structures concerning the presence of pseudoparenchymatous tissue at the base of the synnemata (Réblová et al. 2020a). Our collections (GZCC 19-0463 and GZCC 19-0462) and a Thai specimen (MFLUCC 18-0982) match well with Gamsomyces longisporus in the morphology with overlapping dimensions (Ellis 1976; Dong et al. 2020a; Réblová et al. 2020a). In the molecular analysis (Fig. 50), our isolates and the Thai strain (MFLUCC 18-0982) grouped with Gamsomyces longisporus (CBS 118.86 and CBS 240.89). They formed a sister clade to Gamsomyces stilboideus (MFLUCC 15-0611, MFLUCC 21-0101 and CBS 146494). Sequences of LSU, ITS, SSU, and RPB2 gene regions of G. longisporus taxa revealed high sequence similarity compared to the lower interspecific similarity among Gamsomyces species. We therefore recognize our collections as G. longisporus and treat the Thai collection (MFLUCC 18-0982) (initially described as Pseudobactrodesmium chiangmaiense) as a synonym of G. longisporus.
Gamsomyces malabaricus (Subram. & Bhat) J. Yang & K.D. Hyde, comb. nov.
Index Fungorum number: IF559612; Facesoffungi number: FoF12803.
Basionym: Gangliostilbe malabarica Subram. & Bhat, Kavaka 15: 54 (1987)
Holotype: INDIA. Kerala State, Palghat Dt., Silent Valley, on dead twigs of unidentified plant, D.J. Bhat, 13 July 1980, FFSI No. 4360.
Notes: Gangliostilbe was established by Vittal (1975) with G. indica as the type species. Gangliostilbe and Gamsomyces share synnematous conidiophores and integrated, monoblastic conidiogenous cells. Gangliostilbe malabarica has fusiform, elongated, and light brown conidia, while other species in the genus produce ovoid, obovoid or ellispodial, brown conidia (Vittal 1975; Bhat and Sutton 1985b; Subramanian and Bhat 1987; Marques et al. 2007; Ma et al. 2014). Thus, Gangliostilbe malabarica matches well with the generic concept of Gamsomyces. We therefore propose a new combination Gamsomyces malabaricus. However, we have not examined the holotype of Gamsomyces malabaricus. The details provided by Subramanian and Bhat (1987) are illustrative and descriptive. Synnemata of Gamsomyces malabaricus are much longer (650–950 µm vs. up to 400 µm) than that of Gamsomyces longisporus and Gamsomyces stilboideus (Subramanian and Bhat 1987).
Gamsomyces stilboideus (R.F. Castañeda & G.R.W. Arnold) Hern.-Restr. & Réblová, Stud Mycol 95: 451 (2020)
Index Fungorum number: IF834450; Facesoffungi number: FoF12804; Fig. 52
Gamsomyces stilboideus (MFLU 15-1146) a, b Colony on wood. c Synnema with conidia. d Synnema. e, f Conidiogenous cells. g–m Conidia. n Germinated conidium on PDA medium. o, p Culture on MEA medium, o from above, p from below. Scale bars: a = 500 μm, b = 100 μm, c, d = 50 μm, e, f, m = 30 μm, g–l = 20 μm
Basionym: Bactrodesmium stilboideum R.F. Castañeda & G.R.W. Arnold, Revta Jardín bot Nac Univ Habana 6(1): 48 (1985)
Synonym: Stigmina longispora var. stilboidea (R.F. Castañeda & G.R.W. Arnold) J. Mena & Mercado, Rep. de Rep de Investigacion del Instituto de Ecología y Sistemática, Academia de Ciencias de Cuba, Ser Bot 17: 10 (1987)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Conidiomata on natural substrate synnematous, superficial, scattered, dark brown or mid brown, with glistening conidial masses at the apex, synnemata 160–350 μm long and 16–32 μm wide. Mycelium mostly immersed, composed of septate, smooth, pale brown hyphae. Conidiophores macronematous, fasciculate, erect, straight, or slightly flexuous, cylindrical, smooth, septate, unbranched or branched, mid brown, paler at upper part, tightly compact forming synnemata, thick-walled, 150–350 × 1–3 µm (\({\overline{\text{x}}}\) = 257 × 2.1 µm, n = 20). Conidiogenous cells monoblastic, integrated, terminal, or intercalary, cylindrical, or slightly swollen, pale brown to subhyaline, 8–18 × 2–4 µm (\({\overline{\text{x}}}\) = 11.5 × 2.9 µm, n = 20). Conidia acrogenous, narrowly fusiform, straight, or slightly curved, truncate at the base, rounded to subulate at the apex, 11–13-septate, smooth-walled, guttulate, subhyaline to greenish, paler at the apex, 36–63 × 6–9 µm (\({\overline{\text{x}}}\) = 53.5 × 7.7 µm, n = 35), sometimes with a globose mucilaginous cap at the apex. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on PDA medium within 24 h and germ tubes produced from both ends. Colonies on MEA medium reaching 5–10 mm diam. in a week at 25 °C, in natural light, circular, with velvety, dense, dark brown mycelium on the surface; in reverse yellowish brown in the middle and dark brown at the entire margin.
Material examined: THAILAND, Prachuap Khiri Khan Province, Hua Hin, near Pala-U Waterfall, on decaying wood submerged in a freshwater stream, 25 December 2014, J. van Strien, site4-8-4 (MFLU 15-1146 and HKAS 112585), living cultures MFLUCC 15-0611 and GZCC 15-0058.
Notes: Gamsomyces stilboideus has been reported from Cuba, Mexico, the Philippines, the USA, and Thailand from leaves or submerged twigs (Castañeda-Ruiz and Arnold 1985; Cai et al. 2003a; Heredia et al. 2018; Réblová et al. 2020a). The synnemata of our specimen are smaller (160–350 × 16–32 µm vs. 100–400 × 25–55 µm) and the conidia slightly longer (36–63 × 6–9 µm vs. 30–55 × 7–8 µm) than that of the holotype (Castañeda-Ruiz and Arnold 1985). The LSU, ITS, SSU, and RPB2 sequences of our isolate (MFLUCC 15-0611) and Gamsomyces stilboideus (CBS 146494) showed 100% (833/833 bp), 99.76% (432/433 bp), 100% (1007/1007 bp), and 99.77% (883/885 bp) sequence similarity, respectively.
Sordariomycetes O.E. Erikss. & Winka
Amphisphaeriales D. Hawksw. & O.E. Erikss.
Notes: Amphisphaeriales was introduced by Eriksson (1983) as a provisional order to accommodate Amphisphaeriaceae, Cainiaceae, Clypeosphaeriaceae and Hyponectriaceae and was formally introduced by Hawksworth and Eriksson (1986). Later, Amphisphaeriaceae and related families were placed in Xylariales. The name Amphisphaeriales became out of use (Eriksson and Hawksworth 1993, 1998a, b; Jeewon et al. 2003; Smith et al. 2003; Lumbsch and Huhndorf 2007; Tang et al. 2009). Although Kang et al. (1998) reestablished Amphisphaeriales comprising Amphisphaeriaceae, Cainiaceae and Clypeosphaeriaceae based on phylogeny of 5.8S rDNA and ITS2 sequences, however, it was not well supported (Eriksson 2000; Jeewon et al. 2003). Senanayake et al. (2015) revisited the classification of the subclass Xylariomycetidae and accepted Amphisphaeriales and Xylariales. Based on other collections, morphological traits, and phylogeny, Amphisphaeriales was resurrected with six families including four new ones (Senanayake et al. 2015). However, due to the poor statistical support in Senanayake et al. (2015), Amphisphaeriales was again synonymized to Xylariales (Maharachchikumbura et al. 2016). With further phylogenetic evidence and divergence time estimation, Amphisphaeriales was resolved as a distinct group in Xylariomycetidae with 17 families (Samarakoon et al. 2016; Hongsanan et al. 2017; Hyde et al. 2020a). In our phylogenetic tree inferred from LSU-SSU-TEF1α-RPB2 gene regions (Fig. 53), Amphisphaeriaceae was placed within Xylariales clade.
Maximum likelihood majority rule consensus tree for Sordariomycetes isolates using LSU, SSU, TEF1α, and RPB2 sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated above branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Botryotinia fuckeliana (AFTOL-ID 59) and Dothidea sambuci (DAOM 231303), Exophiala dermatitidis (AFTOL-ID 668) and Pyxidiophora arvernensis (AFTOL-ID 2197). The new collections are in bold with new taxa in blue. Families and orders are indicated with colored blocks. Branches with 100% ML BS and 1.0 PP are thickened
Amphisphaeriaceae G. Winter
Notes: Amphisphaeriaceae has been regarded as a heterogeneous assemblage with 36 genera (Hawksworth et al. 1995). Species in the broadly delimited family are mostly saprobes on wood from terrestrial and aquatic habitats while some are endophytes and pathogens on leaves (Nag Raj 1993; Toofanee and Dulymamode 2002; Wang et al. 2004; Liu et al. 2015a; Senanayake et al. 2015, 2019; Jaklitsch et al. 2016; Luo et al. 2019; Phookamsak et al. 2019; Samarakoon et al. 2019). Based on the morphological revisions and phylogenetic analyses, most genera were excluded from the family and transferred to the related or newly introduced families (Kang et al. 1998, 1999; Kirk et al. 2001; Senanayake et al. 2015). Three genera, Amphisphaeria, Griphosphaerioma and Lepteutypa, were included in the Amphisphaeriaceae (Wijayawardene et al. 2018, 2020; Hyde et al. 2020a). Samarakoon et al. (2020) synonymized Lepteutypa under Amphisphaeria based on the morphological and phylogenetic evaluation.
Amphisphaeria Ces. & De Not.
Notes: Amphisphaeria was introduced by Cesati and de Notaris (1863) with no type species designated. Amphisphaeria umbrina (basionym: Sphaeria umbrina) was later introduced as the lectotype of the genus (Petrak 1923). Among more than 200 epithets in Amphisphaeria, Wang et al. (2004) accepted 12 species after examining 170 type specimens with many having bitunicate asci. Subsequently, seven species were introduced to Amphisphaeria (Marincowitz et al. 2008; Liu et al. 2015a; Phookamsak et al. 2019; Samarakoon et al. 2019; Senanayake et al. 2019; Dissanayake et al. 2020). Samarakoon et al. (2020) reviewed the genus and synonymized Lepteutypa to Amphisphaeria adding three new species and five new combinations. Delineation of Amphisphaeria and Lepteutypa species is difficult due to overlapping morphological characters that do not match well with their phylogeny; thus, the two genera were considered congeneric. Samarakoon et al. (2020) provided an emended description of the genus. Coelomycetous asexual morphs were linked to A. camelliae, A. curvaticonidia and A. sorbi experimentally (Liu et al. 2015a; Samarakoon et al. 2020).
Amphisphaeria uniseptata (C.K.M. Tsui, K.D. Hyde & Hodgkiss) Samarak., Maharachch. & K.D. Hyde, J Fungi 6(3): 20 (2020)
Index Fungorum number: IF836132; Facesoffungi number: FoF08763; Fig. 54
Basionym: Clypeosphaeria uniseptata C.K.M. Tsui, K.D. Hyde & Hodgkiss, Mycologia 93(5): 1004 (2001)
Synonym: Lepteutypa uniseptata (C.K.M. Tsui, K.D. Hyde & Hodgkiss) Jaklitsch & Voglmayr, Persoonia 37: 88 (2016)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 280–330 μm high, 400–450 μm diam., scattered, immersed, erumpent, perithecial, subglobose to conical, dark reddish brown, ostiolate, with a short straight neck. Ostiole periphysate. Ascomatal wall coriaceous, 13–20 μm thick, consisting of multi-layered cells of textura angularis with reddish brown, thick-walled cells to pale brown, thin-walled, elongated cells from outer to inner layer. Paraphyses abundant, persistent, septate, hyaline, tapering towards the apex, 3–4.8 μm wide near the base. Asci 100–135(–190) × 6–8 µm (\({\overline{\text{x}}}\) = 125 × 6.8 µm, n = 30), 90–130 µm long in the sporiferous part, cylindrical, rounded at the apex, 8-spored, pedicellate. Ascospores 14–20 × 3.5–8 µm (\({\overline{\text{x}}}\) = 16.4 × 5.4 µm, n = 40), overlapping, uniseriate, narrowly ellipsoidal, pale brown, uniseptate, smooth-walled, guttulate.
Culture characteristics: Ascospores germinating on WA medium within 24 h. Germ tubes produced from one end. Colonies on PDA medium reaching 5–10 mm diam. after a week at 25 °C in dark, circular, with white and pale yellow mycelium on the surface, forming a dense, velvety, raised ring; in reverse dark brown in the middle and paler towards edge.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS19-2 (HKAS 112607), living culture GZCC 20-0498.
Notes: Amphisphaeria uniseptata was recollected on submerged wood in a freshwater stream in China. Our collection matches with the original diagnosis of A. uniseptata (Tsui et al. 2001c). In the phylogenetic analysis (Fig. 53), this collection clustered with a non-type strain of A. uniseptata (CBS 114967) and an unknown strain of Hypocreomycetidae sp. (M110) with good statistical support. Sequence similarity of our collection and A. uniseptata (CBS 114967) showed one nucleotide difference of LSU and SSU sequences, respectively, and 100% similarity of RPB2 gene region. Comparisons of the available LSU and ITS sequences of Hypocreomycetidae sp. and our specimen revealed 100% similarity. We therefore identified our specimen and Hypocreomycetidae sp. (M110) as A. uniseptata based on morphology and molecular DNA evidence. Hypocreomycetidae sp. (M110) is an endophytic fungus that was isolated from healthy needles of Abies beshanzuensis in Zhejiang Province, China (Yuan et al. 2011). So far, A. uniseptata is only known in China (Guizhou Province, Zhe Jiang Province, and Hong Kong) as a saprobic and endophytic fungus from terrestrial and freshwater habitats.
Amphisphaeria uniseptata is similar to A. micheliae in having immersed, subglobose to oblate, ostiolate ascomata, J+ apical ring of asci and 1-septate, ellipsoidal ascospores. They are slightly different in the dimension and color of ascospores: 14–20 × 3.5–8 µm (\({\overline{\text{x}}}\) = 16.4 × 5.4 µm) vs. 14–19 × 5–7 µm (\({\overline{\text{x}}}\) = 16 × 6 µm) (Tsui et al. 2001c) vs. 15.5–21 × 6–7.5 µm (\({\overline{\text{x}}}\) = 18 × 6.5 µm) (Samarakoon et al. 2020), pale brown vs. yellowish brown.
Annulatascales M.J. D’souza, Maharachch. & K.D. Hyde
Notes: Maharachchikumbura et al. (2015) established Annulatascales to accommodate Annulatascaceae based on an overall Sordariomycetes phylogeny using LSU, SSU, TEF, and RPB2 sequences. Annulatascales is monotypic. Members in the order are saprophytic fungi growing on decaying wood, palm, grass, or bamboo from freshwater and terrestrial habitats (Fröhlich and Hyde 2000; Cai et al. 2002b; Zhang et al. 2017b; Dong et al. 2021a).
Annulatascaceae S.W. Wong, K.D. Hyde & E.B.G. Jones
Notes: Wong et al. (1998a) introduced Annulatascaceae to accommodate six genera reported from tropical rivers and lakes, with Annulatascus as the type genus. A relatively massive, refractive, non-amyloid apical ring was considered as a prominent feature of Annulatascaceae (Wong et al. 1998a). More than 20 genera have been included and referred to the family based on the morphology alone or with phylogenetic analyses (Ho et al. 1999b; Hyde et al. 1999, 2000; Ranghoo et al. 2001; Campbell and Shearer 2004; Vijaykrishna et al. 2005; Zelski et al. 2011; Dong et al. 2021a). Phylogenetic studies indicated that Annulatascaceae is polyphyletic (Ranghoo et al. 1999; Vijaykrishna et al. 2005, 2006; Boonyuen et al. 2011; Luo et al. 2015). Subsequently, several genera were transferred to other families based on molecular data, such as Ascotaiwania and Diluviocola (Ranghoo et al. 1999; Zhang et al. 2017b; Réblová et al. 2018; Luo et al. 2019). Dong et al. (2021a) introduced two new genera to accommodate two Annulatascus species. Hence, 13 genera are accepted in the family, with seven supported by molecular data and forming a monophyletic clade.
Annulatascus K.D. Hyde
Notes: Annulatascus was introduced by Hyde (1992a) based on two freshwater fungi, the type species A. velatisporus and A. bipolaris. Dayarathne et al. (2016) designated an epitype specimen for A. velatisporus and confirmed its systematic placement within Annulatascaceae. The genus is characterized by immersed to superficial, brown, or black ascomata with a long or short neck, unitunicate, cylindrical, pedicellate asci with a relatively massive, refractive apical ring, and mostly uniseriate, hyaline, fusiform ascospores often with appendages or sheaths (Hyde 1992a; Cai et al. 2006; Luo et al. 2015). Annulatascus comprises 18 species and molecular data is available for seven (Chethana et al. 2021; Dong et al. 2021a; Index Fungorum 2022).
Annulatascus tratensis J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF559648, Facesoffungi number: FoF12805, Fig. 56
Etymology: referring to the collecting location in Trat Province, Thailand.
Holotype: MFLU 22-0071.
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 270–550 × 240–520 μm, solitary or gregarious, immersed to semi-immersed, lying obliquely to horizontally, perithecial, subglobose to ellipsoidal, dark brown, ostiolate, with a long straight neck. Ostiole periphysate. Ascomatal wall coriaceous, 28–80 μm thick, 3-layered; outer layer consisting of 3–4 rows of brown, thick-walled, polyhedral cells of textura angularis, middle layer comprising 2–3 rows of dark brown, thick-walled, elongated cells of textura angularis, inner layer comprising 6–7 rows of hyaline, thin-walled, elongated cells of textura angularis or taxtura prismatica. Paraphyses abundant, persistent, septate, hyaline, usually branched, tapering towards the apex, 3–7 μm wide near the base. Asci 185–230(–290) × 10–13 µm (\(\overline{\text{x}}\) = 220 × 11.8 µm, n = 20), cylindrical, with a long pedicel, obtuse at the apex, 8-spored, apex with a relatively massive, refractive, non-amyloid apical annulus, 3–5 µm high, 4.5–7 µm wide. Ascospores 22–32 × 7–13 µm (\(\overline{\text{x}}\) = 27 × 10 µm, n = 40), overlapping, uniseriate, fusiform, straight to slightly curved, hyaline, 0–3-septate, 3-septate when mature, smooth, guttulate, thin-walled, without appendages or a mucilaginous sheath.
Cultural characteristics: Ascospores germinating on PDA medium within 24 h and germ tubes produced from both ends. Colonies on MEA medium, reaching 5–10 mm diam. in 2 weeks at 25 °C, in natural light, circular, with velvety, dense, grayish brown mycelium on the surface; in reverse dark brown with entire margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT22-1 (MFLU 22-0071, holotype; HKAS 112158, isotype), ex-type cultures MFLUCC 17-2055 and GZCC 20-0395; ibid, YJT13-1 (MFLU 22-0067 and HKAS 112151, paratype), ex-paratype cultures MFLUCC 17-2123 and GZCC 20-0387.
Notes: Annulatascus tratensis resembles A. palmietensis and A. tropicalis in having dark brown to black ascomata, cylindrical, pedicellate asci with a large, refractive, non-amyloid apical ring and hyaline, fusiform, 3-septate ascospores lacking bipolar apiculi or appendages and a mucilaginous sheath (Hyde et al. 1998; Tsui et al. 2002). However, they are well distinguished by the dimensions of ascomata, asci, apical ring, and ascospores (Table 2). Annulatascus tratensis has larger ascomata and apical ring than that in A. palmietensis and A. tropicalis (Table 2). Asci of A. tratensis are longer than A. palmietensis and narrower than A. tropicalis (Table 2). Ascospores of A. tratensis are middle-sized among the three species (Table 2). Molecular data is unavailable for A. palmietensis and A. tropicalis. In our phylogenetic analysis, A. tratensis was placed close to A. chiangmaiensis and A. nakhonensis with good statistical support (90% MLBS/0.99 PP) (Fig. 55). Annulatascus chiangmaiensis and A. nakhonensis are distinguished from A. tratensis by the dimensions of ascomata, asci and apical ring, and aseptate ascospores with bipolar apiculi or a mucilaginous sheath (Dong et al. 2021a). The ITS sequence of A. tratensis revealed 97.16% (547/563 bp, five gaps) and 97.12% (539/555 bp, seven gaps) similarity with A. chiangmaiensis and A. nakhonensis, respectively.
Maximum likelihood majority rule consensus tree for Myrmecridiaceae and Annulatascaceae using LSU and ITS sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated near branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Ophiostoma piliferum (AFTOL-ID 910) and Ophiostoma stenoceras (AFTOL-ID 1038). The new collections are in bold and new taxa in blue. Genera are indicated with colored blocks. Ex-type and ex-epitype strains are marked with T and ET after the strain number, respectively. Branches with 100% ML BS and 1.0 PP are thickened
Annulatascus tratensis (MFLU 22-0071, holotype) a Ascomata on natural substrate. b, c Section of ascomata. d Section of peridium. e Paraphyses. f–j Asci. k–n Ascospores. o Apical ring. p Germinated ascospore. q, r Culture, q from above, r from below. Scale bars: a = 500 μm, b, c = 100 μm, f–j = 50 μm, d, e, k, p = 30 μm, l–o = 20 μm
Atractosporales H. Zhang, K.D. Hyde & Maharachch.
Notes: Zhang et al. (2017b) established Atractosporales to accommodate several annulatascaceae-like taxa that previously placed in Annulatascaceae or as genera incertae sedis in Sordariomycetes. Atractosporales initially comprised three families, Atractosporaceae, Conlariaceae, and Pseudoproboscisporaceae (Zhang et al. 2017b). However, in the phylogenetic analyses by Luo et al. (2019) and Hyde et al. (2020a), these families did not form a monophyletic clade. Hyde et al. (2021a) raised Conlariaceae to an order level as Conlariales and retained Atractosporaceae and Pseudoproboscisporaceae in Atractosporales, based on an LSU-SSU-TEF1α-RPB2 phylogeny and molecular clock analysis.
Pseudoproboscisporaceae H. Zhang, K.D. Hyde & Maharachch.
Notes: Based on the phylogenetic and molecular clock analyses, Pseudoproboscisporaceae was introduced to accommodate Pseudoproboscispora, Diluviicola and a Cataractispora species (Zhang et al. 2017b). Later, Dong et al. (2021a) introduced Neodiluviicola to the family with the type species transferred from Diluviicola. Pseudoproboscisporaceae is characterized by immersed to superficial ascomata, often horizontally lying when superficial with a lateral neck, relatively large, non-amyloid apical annulus of asci and fusiform ascospores with bipolar filamentous appendages, which are initially coiled then unfurl becoming long threads (Ho et al. 2004; Zhang et al. 2017b; Dong et al. 2021a). The formation and unfurling mechanisms of ascospores’ appendages are considered the key characteristics to delimit genera in Pseudoproboscisporaceae (Zhang et al. 2017b; Hyde et al. 2020a; Dong et al. 2021a). Asexual morphs are unknown to the family.
Aqualignicola V. M. Ranghoo, K. M. Tsui & K. D. Hyde
Notes: Aqualignicola accommodates freshwater, non-stromatic perithecial ascomycetes with immersed to semi-immersed, hyaline to pale brown, mid brown or dark brown ascomata, with hyaline to brown neck clustered by setae, cylindrical paraphyses tapering towards the apex, cylindrical, pedicellate asci with a distinct, non-amyloid apical annulus and uniseriate, hyaline, ellipsoidal-fusiform, non-septate ascospores with or without a mucilaginous sheath (Ranghoo et al. 2001; Hu et al. 2012). Two species were accepted in the genus with only sexual morphs reported. They were collected on submerged decaying wood in lotic habitats in China and India (Ranghoo et al. 2001; Sudheep and Sridhar 2011; Hu et al. 2012). Because of the lack of sequence data, the systematic placement of Aqualignicola is not confirmed. Regarding its morphology, Aqualignicola was treated as a member of Annulatascaceae and comparable to genera such as Aquaticola, Chaetorostrum, Fluminicola and Longicollum (Ranghoo et al. 2001; Hu et al. 2012; Hyde et al. 2020a). In this study, we identify a new collection as a member of Aqualignicola which clustered within Pseudoproboscisporaceae.
Aqualignicola setosa J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF559649; Facesoffungi number: FoF12806; Fig. 57
Aqualignicola setosa (MFLU 22–0057, holotype) a, b Ascomata on wood. c Section of an ascoma. d Section of peridium. e, f Paraphyses. g Setae. h–m Asci. n–r Ascospores. s, t Apical annuli. u Germinated ascospore. v, w Colonies on PDA medium, v from above, w from reverse. Scale bars: a = 200 µm, b = 100 µm, c = 50 µm, h–m = 30 µm, e, f, n = 20 µm, d, g, s–u = 15 µm, o–r = 10 µm
Etymology: referring to the setose ascomata.
Holotype: MFLU 22-0057
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 120–180 μm high, 90–160 μm diam., scattered or gregarious, semi-immersed to mostly superficial, perithecial, subglobose to conical, dark brown, ostiolate, mostly lying horizontally, with setae 13–40 µm long, 2.2–3 µm wide growing on a cylindrical, laterally upward neck. Ostiole periphysate. Ascomatal wall coriaceous, 16–30 μm thick, 2-layered, outer layer consisting of several rows of dark brown, polygonal, thick-walled cells of textura angularis, inner layer consisting of multi-rows of pale brown to hyaline, polygonal to elongated, thin-walled cells of textura angularis. Paraphyses sparse, persistent, septate, hyaline, tapering towards the apex, 2.5–4 μm wide. Asci (115–)120–160(–170) × 6.5–9 µm (\({\overline{\text{x}}}\) = 138 × 7.9 µm, n = 20), including a stipe 20–42 µm long, cylindrical, obtuse at the apex, 8-spored, with a non-amyloid apical annulus 1.7–2.8 µm wide and 1.2–2.4 µm high. Ascospores 11–17 × 5–6.5 µm (\({\overline{\text{x}}}\) = 13.7 × 5.8 µm, n = 50), arranged obliquely uniseriate, narrowly ellipsoidal, hyaline, aseptate, smooth-walled, guttulate.
Culture characteristics: Ascospores germinating on WA medium within 24 h and germ tube produced from one end. Colonies slow growing reaching 3–5 mm diam. on PDA medium after 2 weeks at 25 °C in natural light, grayish brown to olivaceous brown on the surface; in reverse dark brown.
Material examined: THAILAND, Prachuap Khiri Khan Province, Hua Hin, near Pala-U Waterfall, on decaying wood submerged in a freshwater stream, 25 December 2014, J. van Strien, site4-8-6 (MFLU 22-0057, holotype), ex-type cultures MFLUCC 16-0566 and GZCC 15-0066.
Notes: The morphological characters of Aqualignicola setosa, i.e., brown ascomata with hyaline necks clustered surrounded by setae, non-amyloid apical annulus of cylindrical, pedicellate asci and uniseriate, hyaline, ellipsoidal-fusiform, non-septate ascospores, fit well with the morphology-based concept of Aqualignicola. Aqualignicola setosa can be distinguished from the other two species by the dark brown, mostly superficial, and horizontally orientated ascomata while in A. hyalina they are hyaline to pale brown and semi-immersed, and reddish brown and immersed in A. vaginata. Asci of Aqualignicola species are overlapping in length but different in width with A. hyalina (9–12 µm) wider than A. vaginata (5.5–6.5 µm) and A. setosa (6.5–9 µm) (Ranghoo et al. 2001; Hu et al. 2012). Ascospore dimensions are similar of the three species, A. hyalina 14–15 × 6.25–7.5 µm, A. vaginata 11–15 × 5–6 µm (\({\overline{\text{x}}}\) = 13.4 × 5.3 µm) and A. setosa 11–17 × 5–6.5 µm (\({\overline{\text{x}}}\) = 13.7 × 5.8 µm). The ascospore sheath was described in A. vaginata but not observed in the others.
Without molecular DNA data, Aqualignicola was placed in Annulatascaceae based on morphology. Our four-gene phylogenetic analysis (Fig. 53) revealed Aqualignicola setosa nested within Pseudoproboscisporaceae. The absence of ascospore appendages distinguishes Aqualignicola from other genera in the family. Pseudoproboscisporaceae contains seven strains representing five genera (Réblová et al. 2016a; Dong et al. 2021a; this study). Diluviicola and Neodiluviicola are monotypic genera. Aqualignicola, Cataractispora and Pseudoproboscispora lack sequence data for their type species and some other species in the lateral two genera are retained in Annulatascaceae (Zhang et al. 2017b; Hyde et al. 2020a). Except for Aqualignicola, they all possess filamentous ascospore appendages, initially coiled in ascospore polar chambers or in the conical cap connected to the ascospore or coil-like proboscis, then unfurling to long threads. Due to limited collections, sequence data and the narrow generic delimitation based on coiled morphology and unfurling mechanisms of ascospore appendages, the classification of Pseudoproboscisporaceae taxa is challenging. Asexual morphs are unknown to the family. Thus, morphological and phylogenetic studies of more collections or herbaria specimens, especially the generic type, re-evaluation of the coiled and unfurling mode of ascospore appendages and cultivation studies are needed to resolve the taxonomy of Pseudoproboscisporaceae.
Chaetosphaeriales Huhndorf, A.N. Mill. & F.A. Fernández
Notes: Huhndorf et al. (2004) introduced Chaetosphaeriales to accommodate Chaetosphaeriaceae. Four families are accepted in the order. Hyde et al. (2020a) detailed the classification of newly added taxa in this order.
Chaetosphaeriaceae Réblová, M.E. Barr & Samuels
Notes: Chaetosphaeriaceae was introduced by Locquin (1984) but not validly published. Réblová et al. (1999) formally introduced this family to accommodate Chaetosphaeria and its allies. Chaetosphaeriaceae is a speciose, diverse group of dematiaceous, predominantly phialidic fungi and some non-phialidic, sporidesmium-like and coelomycetous genera (Réblová et al. 2020b; Wu and Diao 2022). Most sexual morphs were classified in Cheatosphaeria. Once the family was introduced, seven sexual genera were included, while more than 80 genera are accepted in the family (Réblová et al. 1999; Wu and Diao 2022). Taxonomic problems of some polyphyletic genera such as Codinaea, Cheatosphaeria, Dictyochaeta and Tainosphaeria have been resolved with new assignments (Réblová et al. 2020b, 2021a, b, c, d, e; Wu and Diao 2022).
Chaetosphaeria Tul. & C. Tul.
Notes: Chaetosphaeria has been placed in various orders and families. Réblová et al. (1999) have reviewed its taxonomic problems in detail. Asexual morphs of Chaetosphaeria are diverse dematiaceous hyphomycetes characterized by phialidic conidiogenous cells (Réblová 2000). Phylogenetic studies were carried out to explain the relationship between Chaetosphaeria and its asexual morphs and indicated Chaetosphaeria was polyphyletic (Réblová 2000; Réblová and Winka 2000; Fernández et al. 2006; Yang et al. 2018c; Lin et al. 2019; Luo et al. 2019). Identifications in Chaetosphaeria are often manifested among the diverse asexual morphs, while the sexual traits show less taxonomic relevance (Réblová 2000; Réblová and Winka 2000).
Chaetosphaeria polygonalis J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559650; Facesoffungi number: FoF12807; Fig. 59
Etymology: referring to the polygonal conidia.
Holotype: HKAS 112570
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, scattered, or aggregated, dark brown, with glistening masses of conidia at the apex. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, solitary or aggregated in small groups, cylindrical, smooth-walled, 3–6-septate, unbranched, dark brown, paler at the apical cell, 50–100 × 2–3.5 µm (\({\overline{\text{x}}}\) = 78.5 × 2.8 µm, n = 25), sometimes arising from creeping hyphae. Conidiogenous cells monophialidic, integrated, terminal, determinate, brown, 24–39 × 2.5–3.5 µm (\({\overline{\text{x}}}\) = 28.3 × 2.9 µm, n = 20), pale brown to subhyaline at the cup-like collarette 2–3 µm wide, 4–7 µm high. Conidia acrogenous, aggregated in slimy masses, hyaline, cuneiform, aseptate, smooth-walled, 1.5–2.5 × 1.2–2 µm (\({\overline{\text{x}}}\) = 1.8 × 1.4 µm, n = 25). Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Colonies on PDA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in dark, with dense grayish brown mycelium in the middle, paler and sparser towards the edge; in reverse white and grayish green in the middle, dark brown in the inner ring and paler at the entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 6 July 2018, J. Yang, SGT1-2 (HKAS 112570, holotype), ex-type cultures MFLUCC 19-0259 and GZCC 20-0438.
Notes: Chaetosphaeria polygonalis was placed as a sister taxon to C. guttulata (Fig. 58). However, they are significantly different in morphology. Chaetosphaeria polygonalis has monophialidic conidiogenous cells and cuneiform, aseptate conidia while C. guttulata has polyblastic conidiogenous cells bearing many tiny protuberant conidiogenous loci and ovoid or fusiform, 3-septate conidia (Luo et al. 2019). Comparisons of the ITS, LSU, SSU, and RPB2 sequences of C. polygonalis and C. guttulata showed 99.58% (472/474 bp), 99.87% (752/753 bp), 100% (886/886 bp), 98.86% (958/969 bp) sequence similarity, respectively. Their TEF1α sequence revealed a low similarity as 89.62% (656/732 bp). We treat C. polygonalis as a distinct species from C. guttulata in the polyphyletic genus Chaetosphaeria.
Maximum likelihood majority rule consensus tree for Chaetosphaeriaceae using ITS, LSU, and TEF1α sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated near branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Tracylla aristata (CBS 141404) and Tracylla eucalypti (CBS 144429). The new collections are in bold and new taxa in blue. Genera and families are indicated with colored blocks. Ex-type and ex-epitype strains are marked as T and ET after the strain number, respectively. Branches with 100% ML BS and 1.0 PP are thickened
Chaetosphaeria polygonalis resembles the asexual morph of C. lentomita (as Chloridium pachytrachelum) and C. dilabens in the morphology of conidiogenous cells and collarettes (Gams and Holubová-Jechová 1976; Réblová and Gams 2000). Chaetosphaeria lentomita and C. dilabens are distinguished from C. polygonalis by the roughly ellipsoidal conidia. Chaetosphaeria bramleyi is similar to C. polygonalis in conidiogenous cells and conidial morphology (Gams and Holubová-Jechová 1976). However, Chaetosphaeria bramleyi has larger conidiophores (25–135 × 4 µm vs. 50–100 × 2–3.5 µm), longer collarette (7–12 µm vs. 4–7 µm) and slightly narrower conidia (1.0–1.5 µm vs. 1.2–2 µm) than those in C. polygonalis (Gams and Holubová-Jechová 1976).
Codinaea Maire
Notes: Codinaea is a phialidic genus with a broad generic concept overlapping with the delimitations of allied genera. The genus is phylogenetically polyphyletic, lacking the molecular data of the type species. Réblová et al. (2021e) conducted a comprehensive study of the genus on its taxonomy, systematics, and biogeography. Codinaea was resolved as a monophyletic clade in Chaetosphaeriaceae comprising four morphotypes and several codinaea-like lineages were recognized and introduced as new genera (Réblová et al. 2021e; Wu and Diao 2022).
Codinaea lignicola (Z.L. Luo, H.Y. Su & K.D. Hyde) Réblová & Hern.-Restr., J Fungi 7(12, no. 1097): 39 (2021)
Index Fungorum number: IF842194; Facesoffungi number: FoF12808; Fig. 60
Basionym: Dictyochaeta lignicola Z.L. Luo, H.Y. Su & K.D. Hyde, Fungal Divers 99: 595 (2019)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, hairy, scattered, or aggregated, brown, with white glistening conidial masses at the apex. Mycelium mostly immersed, composed of septate, smooth, thin-walled, mid brown to pale brown hyphae. Setae fertile, erect, single, straight, or slightly flexuous, cylindrical, slightly tapering towards the apex, smooth-walled, septate, thick-walled, dark brown at the base, paler towards the apex, 180–350 μm (\({\overline{\text{x}}}\) = 270 μm, n = 20) long, 6–12 μm (\({\overline{\text{x}}}\) = 8.3 μm, n = 20) wide near the base, 3–5 μm (\({\overline{\text{x}}}\) = 4 μm, n = 20) wide at the apex. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, cylindrical, smooth-walled, 3–7-septate, unbranched, mid brown at the base, pale brown to hyaline towards the apex, paler than setae, arising in groups (2–6 conidiophores) from the mycelial knots, (50–)70–157(–217) × 3–5 µm (\({\overline{\text{x}}}\) = 105 × 3.8 µm, n = 40). Conidiogenous cells mostly polyphialidic, sometimes monophialidic, integrated, sometimes percurrently proliferating, terminal becoming intercalary, determinate, pale brown to hyaline, with up to 6 funnel-shaped collarettes. Conidia acropleurogenous, aggregated in slimy masses at the apex of conidiophores and setae, allantoid to lunate, aseptate, smooth-walled, guttulate, hyaline, (11–)13–15.5(–17) × 2–4 µm (\({\overline{\text{x}}}\) = 14.7 × 3.2 µm, n = 30), thin-walled, with a 3–8.5 μm long filiform appendage at both ends. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on PDA medium within 24 h and germ tubes produced from one or both ends. Colonies on MEA medium reaching 5–10 mm diam. in a week at 25 °C, in natural light, circular, with velvety, dense, pale-yellow mycelium on the surface, sparser and pale brown at the entire margin; in reverse brown and dark brown.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 6 July 2018, J. Yang, SGT32-1 (HKAS 112577), living cultures MFLUCC 19-0274 and GZCC 20-0454.
Notes: Based on the phylogenetic analysis (Fig. 58), the LSU and ITS sequences of our isolate are identical to the holotype of Codinaea lignicola (DLUCC 0899). However, their morphology are different. The holotype is characterized by mononematous conidiophores and monophialidic or rarely polyphialidic conidiogenous cells without a seta (Luo et al. 2019). However, our collection often has 2–6 conidiophores in groups with a fertile, central seta and polyphialidic, terminal and intercalary conidiogenous cells. Conidiophores of the holotype are longer than that in our collection (204–276 µm vs. 50–217 µm) but shorter than setae (204–276 µm vs. 180–350 μm). Conidia in the holotype are shorter but broader than that in our collection (13–15 × 4.5–5.5 μm vs. 11–17 × 2–4 μm). Both collections were collected from freshwater streams in China. The presence or absence of setae and mono- or polyphialidic conidiogenous cells show less taxonomic value in their identification.
Ellisembia Subram.
Notes: Subramanian (1992) segregated Ellisembia from the speciose, heterogeneous genus Sporidesmium sensu lato based on its distoseptate conidia. However, the subsequent phylogenetic analyses revealed that the euseptate and distoseptate conidia used to delimit sporidesmium-like genera do not appear significant (Su et al. 2016a; Yang et al. 2018a). Based on the evidence from molecular data, Sporidesmium sensu lato species are distributed in various families and orders in Sordariomycetes and Dothideomycetes. Su et al. (2016a) restricted a robust clade with the most sporidesmium-like species in Sordariomycetes as Sporidesmium sensu stricto and synonymized Ellisembia. However, molecular DNA data of the type species of neither Sporidesmium nor Ellisembia is available. Thus, Ellisembia was considered as a separate taxon from Sporidesmium and resolved as polyphyletic (Hyde et al. 2019). We follow the guidelines of Hyde et al. (2019) to use the name Ellisembia for sporidesmium-like species grouped in a monophyletic clade in Chaetosphaeriaceae, some of which possess known sexual morphs.
Ellisembia aquirostrata J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559651; Facesoffungi number: FoF12809; Fig. 61
Etymology: referring to the aquatic habitat of the species and rostrate conidia.
Holotype: HKAS 112610
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, dark brown, scattered or in small groups, glistening. Mycelium mostly immersed. Conidiophores macronematous, mononematous, erect, solitary or caespitose, straight or slightly flexuous, cylindrical, becoming slightly narrower towards the apex, smooth-walled, 1–3-septate, unbranched, dark brown, 35–65 × 3.5–6 µm (\({\overline{\text{x}}}\) = 50 × 4.7 µm, n = 25). Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical, lageniform or ampulliform, pale brown to brown, 9–29 × 3.5–5.5 µm, truncate at the apex, rarely with percurrent proliferations. Conidia acrogenous, fusiform to obclavate, rostrate, subhyaline to pale olivaceous when young, pale brown to brown when mature, apical cells extending into pale brown to hyaline, filiform appendages, basal cell conical-truncate, darker than other cells, 6–8-septate, 41–93 × 10–15 µm (\({\overline{\text{x}}}\) = 60 × 12.5 µm, n = 25), smooth-walled, with a central darkened pore at the septa when mature, sometimes with conidial percurrently proliferations. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h and germ tubes produced from both ends. Colonies growing on PDA medium reaching 10–15 mm in 2 weeks at 25 °C in dark, circular, with dense, yellowish white mycelium on the rough, ring-like surface, darker in the middle; in reverse yellow in the middle and paler at the entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS27-3 (HKAS 112610, holotype), ex-type culture GZCC 20-0503.
Notes: In the phylogenetic analysis of Chaetosphaeriaceae (Fig. 58), Ellisembia taxa clustered in a well-supported monophyletic clade. Species in the clade share brown distoseptate conidia. Ellisembia aurantiaca differs from other species in the clade by reddish brown, ellipsoidal conidia, micronematous or reduced conidiophores and ovoid to pyriform conidiogenous cells (Magyar et al. 2011a). Sexual morphs were reported for Ellisembia aurea and E. foliiculata experimentally which have immersed ascomata, multi-septate, versicolor ascospores and a non-amyloid apical annulus of asci (Réblová and Winka 2001; Hyde et al. 2019). Ellisembia aquirostrata and E. brachypus resemble E. aurea and E. foliiculata in dark brown, macronematous conidiophores and rostrate, obclavate to fusiform conidia with dark conical-truncate basal cells and tapering, paler apical cells. The former two species are distinguished from the latter by central, darkened septal pores and pale brown to hyaline, filiform conidial apical cells. Ellisembia aquirostrata is similar to E. brachypus in morphology. However, Ellisembia aquirostrata has shorter conidiophores than that of E. brachypus reported from various studies (35–65 µm vs. 100–200 µm) (Ellis 1971; Matsushima 1975, 1980, 1993; Shirouzu and Harada 2004; Wu and Zhuang 2005; Abarca et al. 2006; Luo et al. 2019). Comparisons of the LSU and ITS sequences of Ellisembia aquirostrata (GZCC 20-0503) and E. brachypus (HKUCC 10555) revealed 98% (18 bp differences) and 88% (60 bp differences) similarity, respectively.
Ellisembia brachypus is distributed worldwide and recorded in China, Cuba, Ecuador, Japan, India, Kenya, New Zealand, Peru, Sierra Leone, Thailand, and the USA. It has been reported from rotten wood and plant litter of many plant species in freshwater and terrestrial habitats. The conidia of this species have a wide range of variations in different collections. Molecular DNA evidence is needed to identify these remarkably similar collections.
Fuscocatenula Réblová & A.N. Mill.
Notes: Fuscocatenula was segregated from Catenularia based on the distinct phylogenetic placement in Chaetosphaeriaceae. They are similar in morphology but usually differ in conidial maturation (Réblová et al. 2021d).
Fuscocatenula variegata (H.H. Li & X.G. Zhang) Réblová & A.N. Mill., MycoKeys 31: 31 (2021)
Index Fungorum number: IF839470; Facesoffungi number: FoF12810; Fig. 62
Basionym: Catenularia variegata H.H. Li & X.G. Zhang, Mycotaxon 132: 621 (2017)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, hairy, scattered or aggregated, dark brown, with glistening conidial masses or chain-like conidia. Mycelium mostly immersed, composed of brown, septate hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, solitary or aggregated in small groups, cylindrical, smooth, septate, unbranched, dark brown, paler towards the apex, (135–)165–210(–230) × 4–7 µm (\({\overline{\text{x}}}\) = 183 × 5.5 µm, n = 20), constricted at the percurrent portion. Conidiogenous cells monophialidic, integrated, with percurrent proliferating, terminal, determinate, subcylindrical, pale brown to subhyaline, (15–)24–36(–45) × 5–8 µm (\({\overline{\text{x}}}\) = 30 × 6.2 µm, n = 20), with a funneled collarette 4–5 µm wide, 3.2–5 µm high. Conidia acrogenous, arranged in chains, obovoid to pyriform, truncated at the base, aseptate, smooth, guttulate, hyaline to pale brown, 7.5–12 × 5–8 µm (\({\overline{\text{x}}}\) = 10 × 6.7 µm, n = 60). Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Colonies growing on PDA medium reaching 10 mm in a week at 25 °C in dark, circular, with white dense mycelium on the surface; in reverse white with smooth margin, becoming brown from the middle part to the edge with age.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS32-1 (HKAS 112614), living culture GZCC 20-0508.
Notes: Our collection matches with the holotype of Fuscocatenula variegata in morphology and dimensions (Li et al. 2017). However, molecular data is unavailable for the holotype. In the phylogenetic analysis (Fig. 58), our isolate clustered as a sister taxon to the reference specimen of F. variegata (NN055381). In this study, we recollected this species in China and extend its habitat to freshwater.
Kionochaeta P.M. Kirk & B. Sutton
Notes: Kionochaeta is characterized by macronematous, mononematous, dematiaceous, setiform conidiophores with or without lateral, sub-median, fertile or sterile branches, monophialidic, ampulliform, lageniform or cylindrical conidiogenous cells arranged in irregular or penicillate branches at the apex or/and sub-median part of conidiophores, and conidia hyaline, aseptate (rarely 1-septate), ellipsoid, fusiform, falcate or clavate, accumulating in a mucous droplet (Kirk and Sutton 1985; Kuthubutheen and Nawawi 1988). Using DNA sequence data, Lin et al. (2019) confirmed its systematic placement in Chaetosphaeriaceae but was shown to be polyphyletic. Wu and Diao (2022) established Kionochaetiella to accommodate Kionochaeta ivoriensis which was phylogenetically distinct from other Kionochaeta species. Thus, Kionochaeta was resolved as a monophyletic clade. Its sexual-asexual relationship remains unknown.
Kionochaeta castaneae C.G. Lin & J.K. Liu, Mycosphere 10(1): 675 (2019)
Index Fungorum number: IF556707; Facesoffungi number: FoF06288, Fig. 63
Kionochaeta castaneae (HKAS 112575) a Colony on wood. b–e Conidiophores with conidiogenous cells. f–h Conidiogenous cells at the apex of conidiophores. i–l Fertile regions in the middle part of conidiophores. m Conidia. n Germinated conidium. o, p Culture, o from above, p from below. Scale bars: b–e = 50 μm, i–l = 20 μm, f–h = 15 μm, m, n = 10 μm
Saprobic on decaying submerged wood. Asexual morph: Colonies on wood effuse, hairy, scattered or in small groups, brown, with glistening slimy masses of conidia. Mycelium partly superficial, partly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, solitary, setiform, smooth-walled, septate, unbranched, dark brown, pale brown to subhyaline at the apex, (150–)195–235(–290) × 5.5–11 µm (\({\overline{\text{x}}}\) = 212 × 7 µm, n = 20). Fertile region mainly at the middle or lower part and often at the apex of the conidiophore, comprising a compact series of irregular, short branches at the middle loci and less at the apex, 34–63 × 15–40 µm. Conidiogenous cells monophialidic, discrete, terminal, and intercalary, determinate, fascicular, ampulliform or lageniform, smooth-walled, pale brown to subhyaline, 6.5–9 × 2–3.5 µm. Conidia aggregated in slimy masses, hyaline, fusiform or cymbiform, aseptate, smooth-walled, (3.5–)4–6 × 1.2–2 µm (\({\overline{\text{x}}}\) = 5 × 1.5 µm, n = 40), usually guttulate, thin-walled. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium within 24 h. Colonies on PDA medium reaching 5–10 mm diam. in a week at 25 °C in dark, circular, with fluffy, dense, olivaceous mycelium on the surface, sparser and paler at the edge; in reverse ring-like, pale to dark olivaceous with entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 6 July 2018, J. Yang, SGT22-4 (HKAS 112575), living cultures MFLUCC 19-0269 and GZCC 20-0449.
Notes: Our collection is similar to Kionochaeta castaneae in the morphology of terminal and submedian fertile region on the setiform conidiophores with the same conidiogenous cells arrangement (Lin et al. 2019). However, the holotype sometimes produces dark brown, lateral branches from the submedian fertile region which were not observed in our collection. Conidiophores of the holotype are longer than that in our collection [265–320 μm vs. (150–)195–235(–290) μm]. They share the shape and conidial width. However, the holotype has slightly longer conidia than our collection [5.1–6.5 μm vs. (3.5–)4–6 μm]. Although the morphology between our collection and the holotype of K. castaneae is distinct, their sequence data showed high similarity. Comparisons of their ITS, LSU, SSU and TEF1α sequences revealed 99.79% (494/495 bp), 99.87% (819/820 bp), 100% (795/795 bp) and 99.88% (899/900 bp) similarity, respectively. Both specimens were collected from Guizhou Province, China. The holotype was isolated from the decaying shell of a chestnut while our collection was from a submerged decaying twig. We consider them as the same species with molecular evidence following the guidelines of resolving taxonomic ambiguities of Jeewon and Hyde (2016) and expect other collections and genes to confirm their relationship.
Menisporopsis S. Hughes
Notes: Menisporopsis is characterized by macronematous, synnematous conidiophores with a central seta, monophialidic or polyphialidic conidiogenous cells and lunate to falcate, 0–1-septate conidia with one or two (up to 4) setulae at both ends (Hughes 1952; Tsui et al. 1999; Cruz et al. 2014). Eleven species are currently accepted in the genus while two species have been excluded with M. ludoviciana transferred to Vermiculariopsiella and M. novae-zelandiae assigned to a new genus Arcuatospora (Castañeda-Ruiz et al. 1997; Tsui et al. 1999; Cruz et al. 2014; Réblová et al. 2021c). The sexual morph was only reported for M. kobensis based on the cultural study (Matsushima 2003). Menisporopsis theobromae, the generic type, is widespread but lacks molecular data. Liu et al. (2016) designated a reference specimen for M. theobromae and confirmed it as a member of Chaetosphaeriaceae phylogenetically.
Menisporopsis dushanensis C.G. Lin & K.D. Hyde, Mycosphere 10(1): 681 (2019)
Index Fungorum number: IF556710; Facesoffungi number: FoF06291; Fig. 64
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood superficial, effuse, scattered, dark brown, with white, glistening conidial masses at middle lower part of conidiophores. Mycelium mostly immersed, composed of septate, pale brown to hyaline hyphae. Setae central, erect, solitary, straight or slightly flexuous, cylindrical, becoming slightly narrower towards the apex, smooth-walled, septate, brown, paler at the upper part, thick-walled, 300–520 × 6–9 µm, rounded at the apex, lower part encased tightly by compact conidiophores. Synnemata 134–162 µm long, 17–41 µm wide. Conidiophores macronematous, synnematous, tightly compact, appressed to the lower part of setae; single conidiophore straight or slightly flexuous, cylindrical, smooth-walled, septate, unbranched, brown, pale brown at the apical part, 120–162 µm long, 2–4 µm wide. Conidiogenous cells monophialidic, integrated, terminal, determinate, cylindrical, with a small collarette, pale brown, narrower at the apex. Conidia acrogenous, aggregated in slimy masses at the apex of the synnemata, allantoid or lunate, hyaline, aseptate, 14–21 × 2.5–4.5 µm (\({\overline{\text{x}}}\) = 17.5 × 3.6 µm, n = 60), usually guttulate, smooth-walled, thin-walled, mostly with two (rarely three) hyaline, hair-like appendages at both ends of 2.5–11 μm long. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Colonies growing on PDA medium reaching 10 mm in 2 weeks at 25 °C in dark, circular, with rough surface, brown in the middle, with sparse, pale brown to white mycelium at the edge; in reverse brown in the middle and white at the entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS12-1 (HKAS 112604), living culture GZCC 20-0494.
Notes: In the phylogenetic analysis (Fig. 58), our isolate (GZCC 20-0494) clustered with Menisporopsis dushanensis (GZCC 18-0084 and CGMCC 3.20795) with strong support. The LSU and ITS sequences of our isolate (GZCC 20-0494) and the ex-type of M. dushanensis (GZCC 18-0084) showed 100% and 99.04% (5 bp difference) sequence similarity, respectively, while those sequences of M. dushanensis (CGMCC 3.20795) are identical to our isolate. Conidia of the holotype of M. dushanensis produce one to two setulae at both ends while our collection and CGMCC 3.20795 mainly have two setulae at the terminals. Conidia of our collection and the holotype share similar dimensions (14–21 μm long) while those in CGMCC 3.20795 are shorter (11.5–14.5 μm long). Our collection has longer setae than those in the holotype (300–520 μm vs. 207–455 μm) and CGMCC 3.20795 (300–520 μm vs. 370–450 μm), but the dimensions of their synnemata are similar. Based on the guidelines of Jeewon and Hyde (2016), we identify our isolate as M. dushanensis. In this study, we first report M. dushanensis from freshwater habitats on submerged wood while the holotype and CGMCC 3.20795 were from terrestrial leaves (Lin et al. 2019; Wu and Diao 2022).
Neocirrenalia J. Yang & K.D. Hyde, gen. nov.
Index Fungorum number: IF559652; Facesoffungi number: FoF12811
Etymology: referring to the similar genus Cirrenalia.
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, scattered, dark brown, granular, glistening. Conidiophores macronematous, mononematous, erect or arising laterally on hyphae, simple, straight, or flexuous, smooth-walled, septate, unbranched, pale brown to dark brown. Conidiogenous cells monoblastic, integrated, terminal, determinate, subcylindrical, brown. Conidia acrogenous, solitary, regularly helicoid, mostly 1–1.5 times contorted, non-complanate, cells increasing in diameter from base to the terminal, pale brown when young with subglobose to ellipsoidal, paler, and larger terminal cells, dark olivaceous brown to black when mature with opaque septa and contractible terminal cells. Sexual morph: Undetermined.
Type species: Neocirrenalia nigrospora (Somrith., Chatmala & E.B.G. Jones) J. Yang & K.D. Hyde
Notes: Neocirrenalia is introduced as a segregated genus from Cirrenalia to accommodate Cirrenalia nigrospora. Cirrenalia is a heterogeneous genus with several species transferred to phylogenetically distant genera, including Cucurbitinus, Juncigena, Halazoon, Hiogispora, Hydea, and Matsusporium (Abdel-Wahab et al. 2010; Liu et al. 2020). Cirrenalia remains 13 species, some of which are still questionable (Chethana et al. 2021; Index Fungorum 2022). The molecular data is only available for the type species C. macrocephala which belongs to Halosphaeriaceae (Tsui and Berbee 2006; Abdel-Wahab et al. 2010; Hernández-Restrepo et al. 2017; Liu et al. 2020). In comparison, Neocirrenalia is a member of Chaetosphaeriaceae. Conidia of Neocirrenalia nigrospora are black, 1–1.5 times coiled and not constricted at the septa while that in Cirrenalia macrocephala are versicolor, curved to one coiled with strongly constricted septa and a larger and darker terminal cell (Meyers and Moore 1960).
Neocirrenalia nigrospora (Somrith., Chatmala & E.B.G. Jones) J. Yang & K.D. Hyde, com. nov.
Index Fungorum number: IF559653; Facesoffungi number: FoF12812; Fig. 65
Neocirrenalia nigrospora (MFLU 22-0069, reference specimen) a, b Colony on wood. c–f, h, i Conidiophores with conidia. g Conidiophore. j, k Conidia. l Germinated conidium. m, n Culture, m from above, n from below. o–u Sporulated conidia on MEA medium. Scale bars: a, o = 100 µm, b, l = 50 µm, c–k, p–u = 30 µm
Basionym: Cirrenalia nigrospora Somrith., Chatmala & E.B.G. Jones, Nova Hedwig 75(3–4): 479 (2002)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, scattered, dark brown, granular, glistening. Conidiophores macronematous, mononematous, erect or arising laterally on hyphae, simple, straight, or flexuous, smooth, septate, unbranched, pale brown to dark brown, 85–153 µm long, 3–8 µm wide. Conidiogenous cells monoblastic, integrated, terminal, determinate, subcylindrical, brown. Conidia acrogenous, solitary, regularly helicoid, mostly 1–1.25 times contorted, 5–10-septate, non-complanate, cells increasing in diameter from base to the terminal, pale brown when young with subglobose to ellipsoidal, paler and larger terminal cells, dark olivaceous brown to black when mature with opaque septa and contractible terminal cells, basal cells cylindrical, central cells obtusely conical, mature conidial filaments 145–190 µm long, 24–33 µm at the broadest part, spirals 50–70 µm diam. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on PDA medium within 24 h and germ tubes produced from the base. Colonies on MEA medium reaching 5–10 mm diam. in a week at 25 °C, in natural light, circular, with velvety, dense, dark brown mycelium on the surface; in reverse dark brown or black with filiform margin. Hyphae 1.5–2.5 µm wide, septate, branched, pale brown. Conidiophores often obsolete. Conidiogenous cells monoblastic. Conidia acrogenous, solitary, helicoid, 0.5–1 time contorted, 7–11-septate, reddish brown, slightly constricted at the septa, cells increasing in diameter and pigmented from the base to the apex, with a septate filament, mature conidial filaments 90–155 µm long, 15–23 µm at the broadest part, 31–45 µm diam.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT18-3 (MFLU 22-0069, reference specimen; HKAS 112155), living cultures MFLUCC 18-0418 and GZCC 20-0391.
Notes: Our collection matches the morphology and dimensions of Cirrenalia nigrospora except for the erect, typical conidiophores that are not observed in the holotype. Our specimen was collected near the location where the type and other materials of C. nigrospora were found in southern Thailand (Somrithipol et al. 2002). We recognize our collection as Cirrenalia nigrospora and designate a reference specimen for the species. Based on the molecular data, we transfer Cirrenalia nigrospora to a new genus as Neocirrenalia nigrospora. In the phylogenetic analysis (Fig. 58), Neocirrenalia nigrospora formed a distinct branch in Chaetosphaeriaceae sister to Zanclospora. The helicoid conidia of Neocirrenalia nigrospora extend the concept of Chaetosphaeriaceae. Neocirrenalia nigrospora is similar to Cirrenalia donnae and C. longipes in having dark brown or black helicoid conidia with non-constricted septa (Sutton 1973; Ellis 1976; Zhao and Liu 2005a; Zhao et al. 2007). Neocirrenalia nigrospora are effuse, scattered, solitary on the substrates while C. donnae and C. longipes are sporodochial. Neocirrenalia nigrospora has olivaceous brown to black conidia while C. donnae and C. longipes are reddish brown to black. Conidia of N. nigrospora (50–75 µm diam.) and C. longipes (50–75 µm diam.) are of similar size and larger than C. donnae (20–25 µm diam.) (Ellis 1976; Somrithipol et al. 2002; Zhao and Liu 2005a). Mature conidia often coil 1.5–2.5 times in C. longipes and 1–1.25 times in N. nigrospora and C. donnae. The long, septate, conidial filaments of C. longipes are similar to the sporulated, smaller conidia of N. nigrospora in culture (Zhao and Liu 2005a).
As no helicoid fungus has been reported in Chaetosphaeriaceae previously, to avoid mistakes, DNA extraction and sequencing were performed for the second time using a copied isolate with sporulating conidia which showed the same sequence data as the first time.
Nimesporella Réblová & Hern.-Restr.
Notes: Nimesporella was introduced by Réblová et al. (2021e) with a single species N. capillacea. Later, Wu and Diao (2022) described two new species and introduced five new combinations to the genus. Nimesporella possesses mononematous conidiophores, terminal and intercalary, polyphialidic conidiogenous cells extending sympodially and ellipsoidal, fusiform, hyaline, aseptate conidia with a straight or curved setula at each end.
Nimesporella riisgaardii W.P. Wu & Y.Z. Diao, Fungal Divers 116: 187 (2022)
Index Fungorum number: IF841570; Facesoffungi number: FoF12813; Fig. 66
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, scattered or in small groups, dark brown, with glistening white conidial masses at the upper part of conidiophores. Mycelium mostly immersed, composed of septate, pale brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, solitary, straight or slightly flexuous, cylindrical, smooth-walled, septate, unbranched, dark brown, paler towards the apex, guttulate, rounded at the apex, 160–280 × 4.5–6.5 µm (\({\overline{\text{x}}}\) = 210 × 5.4 µm, n = 15). Conidiogenous cells polyphalidic, integrated, sympodial, terminal and intercalary, determinate, pale brown to hyaline, guttulate, with funnel-shaped collarettes. Conidia acropleurogenous, aggregated in slimy masses at the upper half part of the conidiophore, fusiform, hyaline, aseptate, 18–24 × 6.5–10 µm (\({\overline{\text{x}}}\) = 20.6 × 7.9 µm, n = 40), guttulate, smooth-walled, thin-walled, with 6–10 μm long hair-like appendages at both ends. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium within 24 h and germ tubes produced from one or both ends. Colonies on PDA medium reaching 5–10 mm diam. in a week at 25 °C in dark, circular, with velvety, dense, white and grayish green mycelium on the surface, sparser and paler at the edge; in reverse dark grayish green and paler at the entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 6 July 2018, J. Yang, SGT36-1 (HKAS 112579), living cultures MFLUCC 19-0275 and GZCC 20-0456.
Notes: Our collection resembles the holotype of Nimesporella riisgaardii in the morphology and dimensions of the conidiophores, conidiogenous cells and conidia. The intercalary conidiogenous cells of our collection were not observed in the holotype. In this study, Nimesporella riisgaardii is recollected in China and first reported from freshwater habitat.
Oxenbollia W.P. Wu & Y.Z. Diao
Notes: Oxenbollia was introduced by Wu and Diao (2022) to accommodate O. lunatospora. The genus is similar to Codinaeella and Tainosphaeria but phylogenetically distinct (Wu and Diao 2022).
Oxenbollia lunatospora W.P. Wu & Y.Z. Diao, Fungal Divers 116: 190 (2022)
Index Fungorum number: IF841573; Facesoffungi number: FoF12814; Fig. 67
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, hairy, scattered, or aggregated, brown, with glistening conidial masses. Mycelium composed of mostly immersed, hyaline to pale brown, septate hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, cylindrical, narrower at the apex, smooth, septate, constricted at the percurrent portion, unbranched, light brown, paler at the upper part, 36–93(–127) × 2.5–4 µm (\({\overline{\text{x}}}\) = 70 × 3.3 µm, n = 20). Conidiogenous cells mono- or polyphialidic, integrated, with percurrently proliferating, terminal, determinate, subcylindrical, pale brown to subhyaline, 15–37 × 2.5–4.5 µm (\({\overline{\text{x}}} \) = 25.5 × 3.3 µm, n = 15), with 1–4 funnel-shaped collarettes 2.5–4.3 µm wide, 1.5–2.3 µm high. Conidia acrogenous, aggregated in slimy masses, allantoid or cylindrical, gently curved, aseptate, smooth-walled, guttulate, hyaline, 15–21 × 2–4 µm (\({\overline{\text{x}}}\) = 18 × 2.8 µm, n = 20), thin-walled, with a short, 1.2–2.2 μm long filiform appendage at both ends. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h and germ tubes produced from one or both ends. Colonies on PDA medium reaching 10–15 mm in 1 month at 25 °C in dark, with white and grayish brown mycelium on the rough surface; in reverse pale brown in the middle, dark brown in the inner ring and grayish brown at the entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 6 July 2018, J. Yang, SGT4-1 (HKAS 124637), living cultures MFLUCC 19-0261 and GZCC 20-0440; CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS47-1 (HKAS 124631), living culture GZCC 20-0518.
Notes: In the phylogenetic analysis (Fig. 58), our isolates GZCC 20-0440 and GZCC 20-0518 clustered with the ex-type strain of Oxenbollia lunatospora (CGMCC 3.20641) as sister taxa. Comparisons of the ITS and LSU sequences of our isolates and O. lunatospora (CGMCC 3.20641) revealed 98.56% (410/416 bp, two gaps) and 99.72% (719/721 bp) sequence similarity, respectively. Our collections resemble the holotype of O. lunatospora in the morphology and dimensions (Wu and Diao 2022). We therefore recognize our collections as O. lunatospora and extend the habitat of the species to freshwater.
Paragaeumannomyces Matsush.
Notes: Matsushima (2003) introduced Paragaeumannomyces with a single species P. sphaerocellularis which is only known in Japan and lacks molecular data. With similar characteristics to Paragaeumannomyces, eight former Cheatosphaeria species, five new species and four undescribed species were introduced in Paragaeumannomyces (Réblová et al. 2020b). Phylogenetic analyses of combined ITS and LSU sequence data of 35 isolates provided evidence that they formed a well-delimited group in Chaetosphaeriaceae (Réblová et al. 2020a). The emended Paragaeumannomyces possess white, whitish-yellow, ginger-brown to reddish-brown, russet to dark brown ascoma, a unique outer wall of large, thin-walled, globose, subglobose to polyhedral cells with typical chaetosphaeriaceous peridium as inner layers, and scolecosporous, multiseptate, asymmetrical, hyaline to light pink ascospores (Réblová et al. 2020b). Paragaeumannomyces species were linked with a craspedodidymum-like anamorph or chloridium-like synanamorph in cultivation studies (Huhndorf and Fernández 2005; Atkinson et al. 2007; Perera et al. 2016b; Réblová et al. 2020b). A key to Paragaeumannomyces was provided by Réblová et al. (2020b).
Paragaeumannomyces aquaticus J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF900156; Facesoffungi number: FoF12815; Fig. 68
Paragaeumannomyces aquaticus (HKAS 125929, holotype) a Ascomata on wood. b Section of an ascoma. c Section of the apex of peridium. d–f Section of peridium. g Paraphyses. h–k Asci. l–s Ascospores. t Germinated ascospore. u, v Colony on PDA medium, u from above, v from below. Scale bars: b = 50 µm, g–t = 30, c–f = 20 µm
Etymology: referring to the aquatic habitat of the species.
Holotype: HKAS 125929
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 180–280 μm high, 150–260 μm diam., solitary or gregarious, superficial, perithecial, globose to ovoid, roughened, dark brown, papillate, ostiolate. with dark brown, stiff, acute setae, 17–38 × 4.5–8 µm, clustered around ostiole, loosely scattered over entire surface of the ascoma. Ostiole periphysate. Ascomatal wall leathery, 26–48 μm thick, 3-layered; outer layer of textura angularis, 11–24 µm thick, consisting of multi-layered, thin-walled, globose, subglobose to polyhedral, pale brown cells, 6–13 μm diam., grading into smaller cells towards the exterior; middle layer of textura angularis, 13–26 µm thick, composed of multi-layered, thick-walled, polygonal to elongated, smaller, dark brown, melanized cells with setae arising from this layer; inner layer of textura angularis to prismatica, composed of thin-walled, flattened and elongated hyaline cells. Paraphyses numerous, persistent, septate, hyaline, unbranched, tapering towards the apex, 4–5 μm wide near the base. Asci 142–165 × 8.5–13 µm (\({\overline{\text{x}}}\) = 152 × 10.5 µm, n = 20), cylindrical-fusiform, usually curved, with a short pedicel, obtuse at the apex, 8-spored, apex with a small non-amyloid apical annulus. Ascospores 60–73 × 3.5–5 µm (\({\overline{\text{x}}}\) = 66 × 4.3 µm, n = 20), overlapping, filiform, straight, or slightly curved, hyaline, multi-septate, smooth-walled, guttulate, thin-walled, asymmetrical, rounded at the apical end, slightly tapering towards the narrowly rounded basal end.
Culture characteristics: Ascospores germinating on WA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium slow growing, reaching 10–15 mm diam. after 1 month at 25 °C in dark, circular, with velvety, brown mycelium on the surface; in reverse brown with irregular margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 6 July 2018, J. Yang, SGT2-1 (HKAS 125929, holotype), ex-type cultures MFLUCC 19-0260 and GZCC 20-0439.
Notes: In the ITS-LSU-TEF1α phylogenetic analysis (Fig. 58), Paragaeumannomyces aquaticus (GZCC 20-0439) was sister to P. elegans (PDD 118740), and they clustered with P. garethjonesii (MFLU 16-1019). Paragaeumannomyces elegans is distinguished from the new species by having larger ascomata (290–350 μm diam., 280–350 μm high) and asci [(152–)174–221(–227) × 10.5–15(–20)], densely scattered and longer setae (28–60 μm) (Réblová et al. 2020b). Paragaeumannomyces aquaticus is similar to P. garethjonesii and P. panamensis. They share loosely arranged setae over entire ascomata, similar ascomata size, comparable wall thickness and similar length of ascospores. Paragaeumannomyces garethjonesii and P. panamensis differ from the new species in longer setae (38–47 µm and 60–85 µm vs. 17–38 µm), shorter asci (120–152 µm and 120–140 µm vs. 142–165 µm) and narrower ascospores (2.3–3.7 µm and 3–4.1 µm vs. 3.5–5 µm) (Huhndorf and Fernández 2005; Perera et al. 2016b).
The ITS sequence of Paragaeumannomyces aquaticus revealed the highest similarity of 93.82% with P. garethjonesii (MFLUCC 15-1012) among species in the Paragaeumannomyces clade.
Phialoturbella Réblová & Hern.-Restr.
Notes: Réblová et al. (2021c) established Phialoturbella with four species. The type species P. lunata and P. aseptata were initially described in Tainosphaeria (Lin et al. 2019; Luo et al. 2019) and P. calva and P. cecropiae were newly introduced (Réblová et al. 2021c). Sexual morph is known for P. calva and its asexual morph was produced in culture. The genus possesses mononematous, unbranched conidiophores, mono- or polyphialidic conidiogenous with funnel-shaped collarettes and falcate, lunate or oblong, hyaline, aseptate conidia without setulae; sexual morph has superficial, dark brown, papillate ascomata or with a beak-like neck cylindrical-clavate asci with a non-amyloid apical annulus and ellipsoidal, hyaline, aseptate ascospores (Réblová et al. 2021c).
Phialoturbella aquilunata J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559654; Facesoffungi number: FoF12816; Fig. 69
Etymology: referring to the aquatic habitat of the species and the lunate conidia.
Holotype: HKAS 112572
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, hairy, scattered, or aggregated, brown, with glistening conidial masses. Mycelium composed of mostly immersed, hyaline to pale brown, septate hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, cylindrical, narrower at the apex, smooth-walled, septate, sometimes constricted at the septa, unbranched, brown, pale brown at the upper part, 38–83 × 2.5–4 µm (x̅ = 57 × 3.4 µm, n = 25). Conidiogenous cells monophialidic, integrated, sometimes percurrently proliferating, terminal, determinate, subcylindrical, pale brown to subhyaline, 23–38 × 3–4.5 µm (x̅ = 30 × 3.7 µm, n = 20), with a funnel-shaped collarette 2.3–4.3 µm wide, 1.4–2.2 µm high. Conidia acrogenous, aggregated in slimy masses, lunate, gently curved, aseptate, smooth-walled, guttulate, hyaline, 15–24 × 3.5–5.5 µm (x̅ = 20.3 × 4.5 µm, n = 40), thin-walled, with a short and small tip at both ends. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h and germ tubes produced from one or both ends. Colonies growing on MEA medium reaching 10–15 mm in 2 weeks at 25 °C in natural light, with rough surface, white and grayish green in the middle, pale brown at the edge; in reverse dark brown in the middle and brown at the irregular margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 6 July 2018, J. Yang, SGT5-1 (HKAS 112572, holotype), ex-type cultures MFLU 19-0262 and GZCC 20-0441.
Notes: Phialoturbella aquilunata matches the generic concept of Phialoturbella and was phylogenetically placed as a sister taxon to P. aseptata (GZCC 18-0044) with good statistical support (98% MLBS/1.0 PP) (Fig. 58). The ITS and LSU sequences of P. aquilunata differ from P. aseptata by four bp (802/806 bp) and 22 bp (436/458 bp, three gaps), respectively. Conidia of P. aquilunata have an inconspicuous tip at both ends that is lacking in other species of the genus.
Sporoschisma Berk. & Broome
Notes: Sporoschisma is a holomorphic genus with Melanochaeta sexual morphs which was proved by cultural and molecular studies (Hughes 1966; Müller and Samuels 1982c; Goh et al. 1997a; Sivichai et al. 2000; Mugambi and Huhndorf 2008). A chalara-like synanamorph was observed only in vitro (Müller and Samuels 1982c). Sporoschisma remarkably resembles Sporoschismopsis. They share stalked, cylindrical phialidies and brown, septate conidia that develop basipetally and successively in chains. The capitate setae and a swollen venter of phialides are often present in Sporoschisma but absent in Sporoschismopsis. Percurrently regenerating conidiophores were characterized in the latter genus. Conidia of Sporoschisma are cylindrical, mostly truncated at both ends; in Sporoschismopsis they are clavate, obovate or cuneiform with a bluntly rounded apex and truncated base (Goh et al. 1997a; Réblová 2014). Their sexual morphs differ in different types of intrathecal filaments (Samuels and Müller 1978; Müller and Samuels 1982b; Réblová 2014). Réblová (2014) re-evaluated the delimitation of both genera considering the generic changes suggested by Goh et al. (1997a) and proposed to follow their original concepts. The main diagnostic criteria of Sporoschisma and Sporoschismopsis are the presence/absence of capitate setae and conidial anatomy, while the percurrently elongating conidiophores and the shape of the phialide venter show less identification significance. Phylogenetically, Sporoschisma was placed in Chaetosphaeriaceae (Chaetosphaeriales) and Sporoschismopsis in Reticulascaceae (Glomerellales) (Hyde et al. 2020a).
Sporoschisma atroviride J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559655; Facesoffungi number: FoF12817; Fig. 70
Etymology: referring to the dark green conidia.
Holotype: HKAS 112602
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, black, hairy, with glistening conidia in chains. Setae arising from the bulbous base, often with 1–2 at the side of conidiophores, capitate, 1–2-septate, smooth-walled, olivaceous brown, becoming paler towards the apex, straight or slightly flexuous, 100–115 × 4–5.5 µm. Conidiophores macronematous, mononematous, erect, straight or slightly flexuous, solitary or aggregated in small groups, smooth-walled, dark brown to black, composed of a cylindrical stipe, a swollen venter and a phialide stalk, 275–390 µm (\({\overline{\text{x}}}\) = 336 µm, n = 15) long, 17–22.6 µm (\({\overline{\text{x}}}\) = 20.2 µm, n = 15) wide at the tubular collarette, 18–23 µm (x̅ = 22.2 µm, n = 15) wide at venter and 9.5–13.5 µm (\({\overline{\text{x}}}\) = 11 µm, n = 15) wide near the base. Conidiogenous cells monophialidic, integrated, terminal, determinate, dark brown, with a tubular collarette and swollen venter, with flared margin at free end. Conidia 36–49(–53) × (13–)14–15.5(–16) µm (\({\overline{\text{x}}}\) = 42 × 14.7 µm, n = 30), emerging in a chain inside the tubular collarette, develop basipetally, cylindrical, often truncate and sometimes slightly rounded at both ends, terminal conidia usually rounded at the apex, 5–7-euseptate, darkened at the septa, hyaline when young, dark olivaceous brown to dark brown when mature, with pale brown to subhyaline end cells, central cells are longer than the penultimate cells. Sexual morph: Undetermined.
Culture characters: Conidia germinating on WA medium within 24 h and germ tubes produced from both ends. Colonies on PDA medium reaching 7–10 mm diam. after 2 weeks at 25 °C in dark, circular, with velvety, white, sparse mycelium on surface initially, becoming dense, grey from middle to the edge with age; in reverse dark green with white undulate margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS7-1 (HKAS 112602, holotype), ex-type culture GZCC 20-0490; ibid., CS8-1 (HKAS 112603, paratype), ex-paratype culture GZCC 20-0491.
Notes: Among the Sporoschisma species, S. hemipsilum (Hughes 1949), S. longicatenatum (Yang et al. 2016a), S. taitense (Luo et al. 2016b) and S. nigroseptatum (Rao and Rao 1964; Hughes 1966) share the typical Sporoschisma conidiophores with a swollen venter and capitate setae, and cylindrical, 5-septate, versicolor conidia. They can be distinguished by the size of setae and conidiophores, conidial details, and molecular DNA data, except S. nigroseptatum which has not been sequenced. Sporoschisma nigroseptatum differs from the others by the conidia with larger central cells and smaller penultimate cells, while conidial inner cells are almost equal in length of the other three species.
In the original diagnosis of Sporoschisma nigroseptatum, conidia are 5-septate and the third and fourth inner cells are larger than the apical cells (Rao and Rao 1964). Hughes (1966) redescribed this species based on the holotype and other collections, the two conidial central cells are longer, and the penultimate cells are invariably shorter. Latter collections of S. nigroseptatum from various countries matched the conidial anatomy in Hughes (1966). Our collections are remarkably similar to S. nigroseptatum in conidial anatomy. Conidiophores of our collections are longer than the holotype of S. nigroseptatum, and the length of setae is within limits (Rao and Rao 1964; Hughes 1966) (Table 3). Our collections differ from the holotype and other collections of S. nigroseptatum by larger conidia with up to seven septa and wider venter (Table 3). Since the molecular DNA data is unavailable for S. nigroseptatum, we recognize our collections as a separate species from S. nigroseptatum based on the different measurements. However, their relationship needs to be confirmed with molecular evidence.
In the phylogenetic analysis (Fig. 58), our isolates (GZCC 20-0490 and GZCC20-0491) were placed as sister taxa to a non-type strain of S. taitense (KUMCC 15-0241). The ITS sequence of S. atroviride and S. taitense revealed a similarity of 93.34% (505/541 bp, 11 gaps).
Tainosphaeriella Réblová & Hern.-Restr.
Notes: Tainosphaeriella was segregated from Tainosphaeria to accommodate Tainosphaeria aquatica and Tainosphaeria thailandensis based on phylogenetic analyses (Réblová et al. 2021e). The genus is characterized by mononematous, unbranched conidiophores, monophialidic conidiogenous cells with campanulate to almost disk-like collarettes and 1–3-septate conidia with a setula at both ends.
Tainosphaeriella aquatica (X.D. Yu, C.X. Li & H. Zhang) Réblová & Hern.-Restr., J Fungi 7(12, no. 1097): 90 (2021)
Index Fungorum number: IF842224; Facesoffungi number: FoF12818; Fig. 71
Basionym: Tainosphaeria aquatica X.D. Yu, C.X. Li & H. Zhang, Phytotaxa 509: 60 (2021)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, hairy, scattered, or aggregated, brown, with glistening conidial masses. Mycelium composed of mostly immersed, hyaline to pale brown, septate hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, cylindrical, narrower at the apex, smooth-walled, septate, unbranched, light brown, paler towards the apex, 80–140 × 3–4 µm (\({\overline{\text{x}}}\) = 110 × 3.6 µm, n = 20). Conidiogenous cells monophialidic, integrated, sometimes percurrently proliferating, terminal, determinate, subcylindrical, pale brown to hyaline, 28–56 × 3–4.5 µm, with a funnel-shaped collarette 3.2–4 µm wide and 1.5–1.8 µm high. Conidia acrogenous, aggregated in slimy masses, allantoid or cylindrical, gently curved, 0–3-septate, smooth-walled, guttulate, hyaline, (20–)26–32 × 3–4.5 µm (\({\overline{\text{x}}}\) = 27.5 × 3.8 µm, n = 20), thin-walled, with a 19–24 μm long filiform appendage at both ends. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h and germ tubes produced from one or both ends. Colonies on MEA medium reaching 10–15 mm in 2 weeks at 25 °C in natural light, circular, with white mycelium on the surface, wrinkled in the middle; in reverse pale yellow in the middle and white at the entire margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT4-3 (MFLU 22-0063 and HKAS 102157), living cultures MFLUCC 17-2051 and GZCC 20-0373.
Notes: Our collection matches the holotype of Tainosphaeriella aquatica in the morphology of conidia, conidiophores and conidiogenous cells (Li et al. 2021a; Réblová et al. 2021e). Our collection has a similar conidial dimension to the type material but with longer conidiophores (80–140 µm vs. 50–105 μm). Molecular data strongly support the identification of our collection as Tainosphaeriella aquatica which is recollected from Thailand in this study (Fig. 58).
Conioscyphales Réblová & Seifert
Notes: Conioscyphales is a monotypic order established by Réblová et al. (2016c) based on a six-gene phylogenetic study. The order has a close systematic relationship with Pleurotheciales and Savoryellales within the subclass Savoryellomycetidae (Sordariomycetes) (Hongsanan et al. 2017).
Conioscyphaceae Réblová & Seifert
Notes: Conioscyphaceae was introduced to accommodate Conioscypha which was linked to the sexual genus Conioscyphascus based on cultural and molecular studies (Réblová and Seifert 2004; Zelski et al. 2015; Réblová et al. 2016c). Conioscyphaceae species are frequently reported from freshwater habitats (Shearer 1973; Zelski et al. 2015; Chuaseeharonnachai et al. 2017; Luo et al. 2019; Yuan et al. 2020) on decayed wood, leaves, or bamboo stems, while some have been isolated from soil or animal skin and hairs (Udagawa and Toyazaki 1983; Crous et al. 2018c). Sexual morphs of Conioscypha possess immersed to superficial, hyaline to pale orange ascomata with elongated necks, J- apical annulus of asci and hyaline, fusiform to navicular ascospores (Réblová and Seifert 2004; Zelski et al. 2015). Asexual morphs of Conioscypha are characterized by dark brown, globose to subglobose, ellipsoidal or obovoid conidia and cup-shaped conidiogenous cells proliferating percurrently, forming one to several layered collarettes that are the accumulated remnants of the initial outer wall of the conidia (Shearer 1973; Shearer and Motta 1973; Goh and Hyde 1998). The formation of the conidia and collarettes are needed to be confirmed through ultrastructural observation.
In previous phylogenetic studies, Conioscypha species formed a well-supported monophyletic clade in Conioscyphaceae. (Liu et al. 2019a; Luo et al. 2019; Yuan et al. 2020). However, based on molecular DNA data, Yang et al. (2020) confirmed the systematic placement of a Vanakripa species within the Conioscypha clade.
Vanakripa Bhat, Kendrick & Nag Raj
Notes: Bhat and Kendrick (1993) established Vanakripa to accommodate two dematiaceous hyphomycetes with V. gigaspora as the type species. Eleven species are accepted in the genus without known sexual morphs (Index Fungorum 2022). Vanakripa is characterized by sporodochial conidiomata, micronematous or macronematous conidiophores, monoblastic, subcylindrical to vermiform, hyaline conidiophores and 0–1-septate, ellipsoidal, obovoid or pyriform, dark brown conidia. The terminal, subcylindrical to vermiform, hyaline cells were described as conidiogenous cells by Bhat and Kendrick (1993). They are persistently attached to the conidia. Tsui et al. (2003) considered that the “conidiogenous cells” are incapable of proliferating or producing conidia, based on re-examining the type material of V. parva and V. gigaspora. They recognized these cells as the separating cells bearing conidia, referring to similar features in Beltrania, Beltraniopsis and Beltraniella (Pirozynski 1963).
Molecular DNA data is only available for V. minutiellipsoidea and V. chiangmaiense (Vu et al. 2019; Yang et al. 2020; Phukhamsakda et al. 2022). In our phylogenetic analysis (Fig. 53), they positioned within the Conioscypha clade consistent with the result in Yang et al. (2020) and Phukhamsakda et al. (2022).
Vanakripa chiangmaiensis X.G. Tian & Karun., Fungal Diverse 114: 351 (2022)
Index Fungorum number: IF559388; Facesoffungi number: FoF10569; Fig. 72
Saprobic on decaying submerged wood. Asexual morph: Colonies on wood sporodochial, effuse, pulvinate, scattered, black, glistening. Mycelium mostly immersed. Conidiophores micronematous, mononematous, usually undifferentiated, hyaline, simple or sparsely branched, smooth-walled. Conidiogenous cells monoblastic, integrated, terminal, determinate. Separating cells clavate to vermiform, straight, or curved, hyaline, 10–70 × 5–12 µm (\({\overline{\text{x}}}\) = 36.5 × 7.8 µm, n = 30), persistently attached to conidia. Conidia acrogenous, solitary, ellipsoidal to obovoid, aseptate, smooth-walled, guttulate, hyaline to pale grayish green when young, dark grayish green to dark brown when mature, 22.5–30 × 12–21 µm (\({\overline{\text{x}}}\) = 26 × 16.7 µm, n = 45), rounded at the apex, truncate at the base. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium within 24 h. Colonies on PDA medium slow growing, reaching 5 cm diam. in 1 month at 25 °C in dark, circular, floccose, yellowish white, with sparse mycelium on the surface, in reverse yellowish white with undulate margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS43-3 (HKAS 124634), living culture GZCC 20-0516.
Notes: Our collection matches the morphology and dimensions of Vanakripa chiangmaiensis (Phukhamsakda et al. 2022). The LSU, SSU, and ITS sequence data of our isolate and the ex-type strain of Vanakripa chiangmaiensis showed 99.88% (839/840 bp, one gap), 99.8% (996/998 bp) and 99.63% (540/542 bp, one gap) sequence similarity, respectively. Thus, we identify our isolate as V. chiangmaiensis based on their morphology and molecular evidence. We also report a new record of the species in China. In the phylogenetic tree (Fig. 53), V. chiangmaiensis (GZCC 20-0516 and MFLUCC 21-0158) clustered as sister to V. minutiellipsoidea (MFLUCC 17-2366 and CBS 112523) within Conioscypha clade. We follow Yang et al. (2020) and Phukhamsakda et al. (2022) to treat Vanakripa and Conioscypha as separate genera based on their morphology. Sexual morphs were described for three Conioscypha species while they are unknown to Vanakripa. Further collections with molecular data and the discovery of sexual morphs may assist in resolving the taxonomy between Vanakripa and Conioscypha.
Conlariales K.D. Hyde & Hongsanan
Notes: Based on the phylogenetic and molecular clock analyses, Hyde et al. (2021a) established the monotypic order Conlariales to accommodate Conlariaceae.
Conlariaceae H. Zhang, K.D. Hyde & Maharachch.
Notes: Conlariaceae was initially placed within Atractosporales with a single genus Conlarium (Zhang et al. 2017b). Later phylogenetic studies proved that Riomyces rotundus positioned close to Conlarium and recognized as a member of Conlariaceae (Luo et al. 2019; Hyde et al. 2020a). However, like the case of Vanakripa and Conioscypha, the phylogenetic placement of Riomyces rotundu is problematic as it nested within Conlarium, but the two genera can be distinguished morphologically (Yuan et al. 2020; Dong et al. 2021a). Conlarium is characterized by partially immersed to superficial ascomata with a long neck, branched and septate paraphyses, conspicuous apical ring of asci and biseriate, hyaline, fusiform, 0–5-septate ascospores with or without globose or papillary appendages at one or both ends (Liu et al. 2012). Riomyces has immersed to erumpent ascomata with a short neck, hamathecium with globose, deliquescent cells, asci without apical apparatus and overlapping uniseriate, hyaline, broadly ellipsoidal-fusiform, 3-septate ascospores surrounded by a gelatinous sheath (Ferrer et al. 2012). Asexual morphs of Conlarium are monodictys-like taxa while is unknown in Riomyces. Additional collections and descriptions of asexual morphs are needed to resolve the phylogenetic relationship between Conlarium and Riomyces.
Conlarium F. Liu & L. Cai
Notes: Conlarium comprises eight saprobic, lignicolous species from freshwater and terrestrial environments or endophytes on sugarcane rhizosphere from China and Thailand (Liu et al. 2012; Zhang et al. 2017b; Phookamsak et al. 2019; Xie et al. 2019; Dong et al. 2021a; Dubey and Manikpuri 2021). Conlarium dupliciascosporum, the generic type, is the only holomorphic species in the genus. The remaining species are monodictys-like asexual morphs from natural substrates or cultures.
Conlarium muriforme J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559656; Facesoffungi number: FoF12819; Fig. 74
Etymology: referring to the muriform conidia.
Holotype: HKAS 112573
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, sporodochial, broadly punctiform, dark brown, scattered, glistening. Mycelium mostly immersed, composed of septate, smooth, brown to hyaline hyphae. Conidiophores micronematous to semi-macronematous, mononematous, erect, hyphae-like, often flexuous, septate, smooth-walled, pale brown to subhyaline, 2–3.5 µm wide. Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical, pale brown to subhyaline. Conidia acrogenous, solitary, subglobose, ellipsoidal, or irregular, muriform, clathrate, smooth-walled, subhyaline to pale brown when young, dark brown when mature, 17–35 × 16–34 µm (\({\overline{\text{x}}}\) = 26.6 × 23.7 µm, n = 40), constricted at the septa. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h and germ tubes produced from basal cells. Colonies on PDA medium slow growing, reaching 10–15 mm in 1 month at 25 °C in dark, circular, producing brown pigment, with velvety, grayish brown mycelium on the surface; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying twig submerged in a freshwater stream, 6 July 2018, J. Yang, SGT7-2 (HKAS 112573, holotype; HKAS 125931, isotype), ex-type culture GZCC 20–0442.
Notes: Conlarium muriforme was isolated from submerged decaying wood in a freshwater stream. Among Conlarium species, C. baiseense, C. nanningesnse and C. sacchari are endophytes described from sporulating cultures. They are distinguished from Conlarium muriforme (from the natural substrate) by conidial color, size, and cell number (Xie et al. 2019). Conlarium muriforme differs from C. aquaticum and C. thailandense by smaller conidia [17–35 × 16–34 µm (\({\overline{\text{x}}}\) = 26.6 × 23.7 µm) vs. 45–70 × 20–57 µm (\({\overline{\text{x}}}\) = 54.9 × 39.3 µm) vs. 25–45 × 17–33 µm (\({\overline{\text{x}}}\) = 35.9 × 26.9 µm)] (Zhang et al. 2017b; Phookamsak et al. 2019). Conlarium muriforme (GZCC 20–0442) clustered as a sister taxa to C. baiseense (HMAS 247298 and HMAS 247986) (Fig. 73). The ITS, LSU, and SSU sequences of Conlarium muriforme differ from C. baiseense by 28 bp (474/502 bp, six gaps), two bp (835/837 bp) and two bp (961/963 bp), respectively.
Maximum likelihood majority rule consensus tree for Conlarium using LSU, ITS, and SSU sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated near branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Lentomitella tenuirostris (CBS 138734) and Lentomitella magna (ICMP 18371). The new taxa are in blue and ex-type strains are marked with T after the strain number. Branches with 100% ML BS and 1.0 PP are thickened
Fuscosporellales J. Yang, J. Bhat & K.D. Hyde
Notes: Fuscosporellales is a monotypic order with Fuscosporellaceae as the type family (Yang et al. 2016b). Based on the phylogenetic and molecular clock analyses, it was placed within Savoryellomycetidae with Conioscyphales, Pleurotheciales and Savoryellales (Yang et al. 2016b; Hongsanan et al. 2017; Hyde et al. 2020a).
Fuscosporellaceae J. Yang, J. Bhat & K.D. Hyde
Notes: Fuscosporellaceae comprises two sexual genera Plagiascoma and Pseudoascotaiwania and four asexual genera Bactrodesmiastrum, Mucispora, Fuscosporella and Parafuscosporella. The family is characterized by immersed to semi-immersed ascomata, papillate or with a rostrate neck, non-amyloid apical annulus of asci, uniseriate, septate, fusiform, hyaline, or versicolor ascospores; micronematous to macronematous conidiophores, monoblastic conidiogenous cells and brown, septate, ellipsoidal to obovoid, pyriform or obpyramidal conidia (Fallah et al. 1999; Boonyuen et al. 2016; Réblová et al. 2016c; Yang et al. 2016b, 2017).
Fuscosporella J. Yang, J. Bhat & K.D. Hyde
Notes: Fuscosporella is similar to Parafuscosporella in having sporodochial conidiomata, monoblastic, hyaline, subglobose to ellipsoidal conidiogenous cells and dark brown, ellipsoidal to pyriform conidia. Parafuscosporella species always have a jelly-like cover of the conidiomata, which was not observed in Fuscosporella (Boonyuen et al. 2016; Yang et al. 2016b, 2020). They are distinguished by their conidial morphology in culture and are phylogenetically separated.
Fuscosporella atrobrunnea L.L. Liu, J. Yang & Z.Y. Liu, sp. nov.
Index Fungorum number: IF559707; Facesoffungi number: FoF12820; Fig. 75
Etymology: referring to the dark brown conidia.
Holotype: GZAAS 20-0435
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates sporodochial, scattered, dark brown to black, glistening. Mycelium partly immersed, partly superficial, composed of septate, pale brown to hyaline hyphae. Conidiophores semi-macronematous, mononematous, hyaline, smooth-walled, 2–4 μm wide. Conidiogenous cells monoblastic, integrated, terminal, globose, subglobose, ellipsoidal or vermiform, hyaline, 16–39 × 9.5–16 μm (\({\overline{\text{x}}}\) = 24.5 × 12.5 μm, n = 30). Conidia acrogenous, lying obliquely to horizontally, smooth-walled, broadly ellipsoidal, or somewhat irregular, dark brown, 32–39 × 20–25 μm (\({\overline{\text{x}}}\) = 35.5 × 23 μm, n = 30), with two tightly compact, hyaline to brown, papillate cells at both ends, the base bearing conidiogenous cells, another end sometimes bearing a hyaline, globose to vermiform cell. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm diam. after 1 month at 25 °C in dark, circular, matte, with dense, velvety, grayish brown mycelium on the surface, becoming ring-like, dark, and grayish brown; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, 28° 25′ N, 106° E, elevation 500 m, on decaying twig submerged in a freshwater stream, 16 July 2019, L.L. Liu, CS1-7 (GZAAS 20-0435, holotype), ex-type culture GZCC 19-0540.
Notes: Molecular evidence confirmed that Fuscosporella atrobrunnea (GZCC 19-0540) grouped as sister to F. pyriformis (MFLUCC 16-0507) and F. aquatica (MFLUCC 16-0859) with strong statistical support (100% MLBS/1.0 PP) (Fig. 53). Fuscosporella pyriformis is distinguished from the other two species by uniseptate pyriform conidia with a pale brown, obconical basal cell. Fuscosporella atrobrunnea is similar to F. aquatica in having dark brown, broadly ellipsoidal or somewhat irregular conidia with papillate, paler cells at both ends. The basal cells bear hyaline, subglobose to vermiform conidiogenous cells and the apical cells have potential to produce a cell that resembles the conidiogenous cells. Fuscosporella atrobrunnea has smaller conidia than F. aquatica (32–39 × 20–25 μm vs. 38–60 × 25.5–37 μm) (Yang et al. 2017). Conidial septa of F. atrobrunnea were not observed due to the intense pigmentation of conidia, and further ontogeny study is needed to resolve the mechanism of conidial development.
Glomerellales Chadef. ex Réblová, W. Gams & Seifert
Notes: “Glomérellales” was introduced by Chadefaud (1960) for plant endophytes and parasites comprising Gibellina, Glomerella, Phyllachora, Physalospora and Polystigma. Glomerellaceae, the type family, was introduced by Locquin (1984). However, the order and family were invalidly published without a Latin diagnosis. With molecular DNA data, Glomerellaceae was validated by Zhang et al. (2006) as incertae sedis in Hypocreomycetidae. Subsequently, Glomerellales was phylogenetically delimited and validated to include three families, Glomerellaceae, Australiascaceae, and Reticulascaceae (Réblová et al. 2011a). Maharachchikumbura et al. (2015) and Tibpromma et al. (2018) added Plectosphaerellaceae and Malaysiascaceae to the order.
Australiascaceae Réblová & W. Gams
Notes: Australiascaceae is a monotypic family with the holomorphic genus Monilochaetes as the type (Réblová et al. 2011a). Sivanesan and Alcorn (2002) erected Australiasca with type species A. queenslandica which was linked to the asexual morph Dischloridium camelliae experimentally. Australiasca queenslandica is similar to members of Chaetosphaeriaceae by the traits of ascomata, asci, ascospores and phialidic asexual morphs, while its gemination type resembles Lasiosphaeriaceae (Sivanesan and Alcorn 2002). Based on a phylogenetic study, Australiasca was confirmed unrelated to Chaetosphaeriaceae and Lasiosphaeriaceae but congeneric with the asexual genus Monilochaetes (Réblová et al. 2011a). Thus, Dischloridium was synonymized to Monilochaetes. Following the rule of the ICN, Monilochaetes was recommended for use rather than Australiasca based on its priority and fewer name changes (Réblová et al. 2016b).
Monilochaetes Halst. ex Harter
Notes: Monilochaetes is characterized by superficial, dark brown, setose or glabrous ascomata with a short beak and hyaline, sub-ellipsoidal ascospores becoming 1–3-septate after discharge; and macronematous, mononematous conidiophores, monophialidic conidiogenous cells and hyaline, cylindrical, ellipsoid or narrowly obovoid conidia, often aseptate. The genus contains ten species with two having sexual morphs (Sivanesan and Alcorn 2002; Réblová et al. 2011a; Chethana et al. 2021; Index Fungorum 2022). Eight species are available with molecular data, except M. basicurvata and M. regenerans. They formed a well-defined monophyletic clade and were distinct from the similar genus Exochalara (Leotiomycetes) and the phialidic species in Chaetosphaeriaceae. Synanamorphs with microconidia were observed in cultures of M. camelliae, M. dimorphospora and M. nothapodytis (Réblová et al. 2011a, b; Zhou et al. 2017).
Monilochaetes species were isolated from fresh or dead leaves, stipes, stems, twigs, or branches of various plants. Monilochaetes infuscans, the generic type, is pathogenic to the sweet potato (Ipomoea) causing scurf disease (Halsted 1890; Harter 1916). Monilochaetes nothapodytis is a plant endophyte of Nothapodytes pittosporoides (Zhou et al. 2017). Monilochaetes melastomae was isolated from spots on fresh leaves and might be a plant pathogen (Crous et al. 2018c). An unknown Monilochaetes species was isolated from the sludge at a sewage outlet of the pesticide factory with cypermethrin as the only carbon source (Qin et al. 2010). The Monilochaetes species in this study is the first report of the genus from a freshwater habitat.
Monilochaetes alsophilae J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559708; Facesoffungi number: FoF12821; Fig. 76
Monilochaetes alsophilae (HKAS 112601, holotype) a, b Colony on wood. c, d Conidiophores with conidia. e, f Conidiophores. g, h Conidiogenous cells. i–k Conidiogenous cells with conidia. l, m Conidia. n Germinated conidium. o, p Culture, o from above, p from below. Scale bars: c–f = 150 μm, g–n = 30 μm
Etymology: referring to the host genus Alsophila.
Holotype: HKAS 112601
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, scattered or aggregated, dark brown, with glistening masses of conidia or chain-like conidia at the apex. Mycelium mostly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, solitary or aggregated in small groups, cylindrical, tapering towards the apex, smooth-walled, septate, unbranched, dark brown, pale brown to subhyaline at the upper part, 500–800 µm long, 13–28 µm wide near the base. Conidiogenous cells monophialidic, integrated, usually elongating percurrently with a swallow part, terminal, determinate, subcylindrical, pale brown to subhyaline, 36–62 × 6–10 µm, with a funneled collarette. Conidia acrogenous, aggregated in slimy masses or in chains, cylindrical-ellipsoidal, hyaline, broadly rounded at both ends, slightly narrower at the middle part, aseptate, smooth-walled, 23–27 × 11–15 µm (\({\overline{\text{x}}}\) = 24.5 × 12.5 µm, n = 35), guttulate, thin-walled. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Colonies growing on PDA medium reaching 10–15 mm in a week at 25 °C in dark, with dark brown, velvety mycelium on the surface; in reverse dark brown to black with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on dead stipes of Alsophila spinulosa submerged in a freshwater stream, 11 July 2019, J. Yang, CS1-1 (HKAS 112601, holotype; HKAS 125923, isotype), ex-type culture GZCC 20-0486; CS4-1 (HKAS 124628, paratype), ex-paratype culture GZCC 20-0487.
Notes: Monilochaetes alsophilae was isolated from submerged stipes of the tree fern Alsophila spinulosa growing at the waterside. In the phylogenetic tree inferred from LSU, SSU, TEF1α, and RPB2 gene regions, Monilochaetes alsophilae (GZCC 20-0486 and GZCC 20-0487) clustered within Monilochaetes as sister taxa to M. pteridophytophila (MFLUCC 21-0022) with strong statistical support (100% MLBS/1.0 PP) (Fig. 53). Comparisons of the LSU, ITS, SSU, TEF1α, and RPB2 sequences of M. alsophilae and M. pteridophytophila revealed 99.89% (877/878 bp), 96.74% (356/368 bp), 99.80% (998/1000 bp), 97.27% (891/916 bp) and 94.35% (986/1045 bp) similarity, respectively. Monilochaetes alsophilae and M. pteridophytophila were isolated from stipes or stalks of Alsophila (Zhang et al. 2021; this study). However, M. alsophilae was collected from a stream in China while M. pteridophytophila from a forest in Thailand (Zhang et al. 2021). They are similar in morphology and conidial dimensions. Monilochaetes alsophilae is distinguished by longer conidiophores (500–800 µm vs. 268–565 µm). In addition, conidiophores of M. alsophilae are conspicuously longer than other species in the genus (500–800 µm vs. up to 640 µm). Monilochaetes alsophilae resembles M. laeensis and M. nothapodytis in having aseptate, cylindrical-ellipsoidal conidia with broadly rounded ends and similar size. Monilochaetes laeensis has a basal conidial scar which was not observed in M. nothapodytis and the new species. Conidia of M. alsophilae (23–27 × 11–15 µm) are slightly larger than M. laeensis (22–26 × 10–12 μm) and M. nothapodytis (16.5–24 × 9.5–15.5 μm in vitro) (Réblová et al. 2011a; Zhou et al. 2017). The formation of microconidia was observed in the two latter species, which needs further study.
Reticulascaceae Réblová & W. Gams
Notes: Reticulascaceae was established to accommodate two holomorphic genera Reticulascus (synonym: Cylindrotrichum) and Porosphaerellopsis (synonym: Sporoschismopsis) and an asexual genus Kylindria, with well-supported molecular data (Réblová et al. 2011a). The sexual-asexual connections were proved by cultural and molecular studies (Réblová and Gams 1999; Réblová et al. 2011a; Réblová 2014). The asexual names Cylindrotrichum and Sporoschismopsis take priority and are widely used over their sexual names. Hyde et al. (2016b) described a new species Blastophorum aquaticum in the family, but later reclassified in Cylindrotrichum (Luo et al. 2019).
Kylindria DiCosmo, S.M. Berch & W.B. Kendr.
Notes: Kylindria was segregated from Cylindrotrichum to accommodate five Cylindrotrichum species (DiCosmo et al. 1983). Kylindria is characterized by monophialidic conidiogenous cells often swollen at the upper part with or without a collarette and occasionally elongating percurrently, cylindrical or ellipsoidal, 0–3-septate (mostly 3) conidia, with or without an eccentric protruding hilum near the base (DiCosmo et al. 1983; Bhat and Sutton 1985a; Rambelli and Onofri 1987; Castañeda-Ruíz 1988; Matsushima 1993; Zhang et al. 2010; Maharachchikumbura et al. 2018; Luo et al. 2019). Cylindrotrichum has seta-like conidiophores, mono- or polyphialidic conidiogenous cells without elongation and cylindrical or ellipsoidal, 0–1-septate, rarely 3-septate conidia (Gams and Holubová-Jechová 1976; DiCosmo et al. 1983; Rambelli and Onofri 1987; Arambarri and Cabello 1989; Holubová-Jechová 1990; Maharachchikumbura et al. 2018; Luo et al. 2019). The type species of Cylindrotrichum is holomorphic and its systematic position was supported by molecular DNA data (Réblová et al. 2011a). However, Kylindria lacks known sexual morphs and sequence data is unavailable for its generic type. Three sequenced Kylindria species clustered close to Cylindrotrichum but are distinct (Maharachchikumbura et al. 2018). We follow Réblová et al. (2011a) and recognize both genera.
Kylindria peruamazonensis Matsush., Matsush. Mycol Mem 7: 56 (1993)
Index Fungorum number: IF360885; Facesoffungi number: FoF12822; Fig. 77
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates effuse, hairy, scattered, brown, with glistening conidial masses at apex. Mycelium partly superficial, partly immersed, composed of septate, smooth, pale brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, solitary, straight or slightly flexuous, cylindrical, smooth-walled, septate, unbranched, dark brown or mid brown, becoming paler towards the apex, 210–270 × 5–6 µm, 7–10 µm wide near the base. Conidiogenous cells monophialidic, integrated, terminal, long lageniform, broader at the upper part and narrower at the apex, pale brown, 40–49 × 7.5–9 µm. Conidia acrogenous, aggregated in slimy masses, cylindrical-ellipsoidal, hyaline, aseptate when immature, 3-septate at maturity, smooth-walled, guttulate, 19–26 × 6–8 µm (\({\overline{\text{x}}}\) = 21.8 × 7 µm, n = 20), with an excentric, lateral protuberance of the basal cell. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h. Germ tubes produced from one or both ends. Colonies on MEA medium reaching about 10 mm diam. after 2 weeks at 25 °C in natural light, circular, with mid brown, velvety mycelium on the surface and sparse, pale brown at the edge; in reverse dark brown in the middle colorless at the entire margin.
Material examined: THAILAND, Phang Nga Province, Bann Tom Thong Khang, on decaying wood submerged in a freshwater stream, 17 December 2015, J. Yang, site7-37-1 (MFLU 22-0060 and HKAS 112183), living cultures MFLUCC 16-0871 and GZCC 17-0026.
Notes: Kylindria peruamazonensis was described from a sporulating culture isolated from a palm petiole in Peru. The two strains of Kylindria peruamazonensis (CBS 838.91 and CBS 421.95) were identified through cultural morphology that matched the original diagnosis (Réblová et al. 2011a). Our collection was described from decaying submerged wood. It matches K. peruamazonensis in morphology but has longer conidiophores (210–270 µm vs. 50–125 µm) and slightly larger conidia (19–26 × 6–8 µm vs. 12.5–23 × 4–7.5 µm) than the holotype in culture (Matsushima 1993). In the phylogenetic analysis (Fig. 53), our isolate (MFLUCC 16-0871) clustered with two strains of K. peruamazonensis (CBS 421.95 and CBS 838.91) and was distinct from other Kylindria species. The ITS and LSU sequences of our collection and K. peruamazonensis (CBS 421.95 and CBS 838.91) revealed one nucleotide difference, respectively. We therefore recognize our collection as K. peruamazonensis based on the molecular evidence and morphological characters.
Kylindria peruamazonensis is similar to K. chinensis, K. excentrica and the type species K. triseptata in having long lageniform conidiogenous cells and 3-septate, cylindrical-ellipsoidal conidia with laterally displaced hilum (Matsushima 1975, 1993; Bhat and Sutton 1985a; Réblová et al. 2011a; Maharachchikumbura et al. 2018). This kind of conidia was occasionally found in K. triseptata while it mostly has conidia with a tapering basal part and truncate base (Matsushima 1975). Phialides with collarettes are present in K. chinensis and K. peruamazonensis but lacking in K. excentrica and K. triseptata. Conidia of K. excentrica (27.5–35 × 7.5–8.5 µm) are larger than that in K. chinensis (17–23 × 5–7 µm), K. peruamazonensis (19–26 × 6–8 µm) and K. triseptata (18–24 × 6–7.5 µm) (Bhat and Sutton 1985a; Matsushima 1975; Maharachchikumbura et al. 2018this study).
Kylindria peruamazonensis was initially isolated from palm petioles in Peru (Matsushima 1993). It has been reported on plant pollen, in rainwater and river water in Poland (Czeczuga and Orłowska 1999, 2001; Czeczuga et al. 2003) and leaf litter of Bucida palustris in Cuba (Réblová et al. 2011a). In this study, we report K. peruamazonensis from a freshwater stream and as a new record in Thailand.
Hypocreales Lindau
Notes: Based on the phylogenetic analyses and divergence time estimation, 14 families and 303 genera are accepted in Hypocreales (Hyde et al. 2020a).
Tilachlidiaceae L. Lombard & Crous
Notes: Lombard et al. (2015) established Tilachlidiaceae to accommodate two asexual genera Septofusidium and Tilachlidium based on molecular DNA data. Later, Pawłowska et al. (2017) introduced a sexual genus Psychronectria in the family.
Uvarisporella J. Yang, Jian K. Liu & K.D. Hyde, gen. nov.
Index Fungorum number: IF559709; Facesoffungi number: FoF12823
Etymology: referring to the grape-like conidial mass.
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates effuse, scattered, brown, glistening, with masses of conidia at the apex of conidiophores. Mycelium mostly immersed, composed of septate, smooth-walled, pale brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, slightly curved, solitary, cylindrical, smooth-walled, septate, unbranched, brown. Conidiogenous cells polyblastic, integrated, terminal, determinate, brown to pale brown, cylindrical or swollen, percurrently proliferating, with the apex becoming swollen and new conidiogenous cells formed enteroblastically then breaking through the outer wall, often remaining the lower flared fragments that not attached to the new conidiogenous cell. Conidia acrogenous, clustered in masses, globose, subglobose or obovoid, uniseptate, brown, guttulate, thick-walled, truncate or concave at the base with a minute marginal frill. Sexual morph: Undetermined.
Type species: Uvarisporella aquatica J. Yang, Jian K. Liu & K.D. Hyde
Uvarisporella aquatica J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559710; Facesoffungi number: FoF12824; Fig. 78
Uvarisporella aquatica (HKAS 112622, holotype) a, b Colony on wood. c–g, m Conidiophores and conidia. h, i Conidiophores and conidiogenous cells. j Monoblastic conidiogenous cell. k Conidiogenous cell with percurrent proliferation. l, n Swollen polyblastic conidiogenous cells with conidia. o–q Conidia. Scale bars: c–f, h, i, l, m = 50 μm, g, j, k, n, o = 30 μm, p, q = 20 μm
Etymology: referring to the aquatica habitat of the species.
Holotype: HKAS 112622
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates effuse, scattered, brown, glistening, with masses of conidia at the apex of conidiophores. Mycelium mostly immersed, composed of septate, smooth, pale brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly curved, solitary, cylindrical, smooth-walled, septate, unbranched, mid brown to dark brown, often paler at the apex, 60–300 µm long, 5–9 µm wide near the base. Conidiogenous cells mono- to polyblastic, integrated, terminal, determinate, brown to pale brown, cylindrical or swollen ellipsoidal after regenerating, when percurrently proliferating, the apex becoming swollen, new conidiogenous cells formed enteroblastically then breaking through the outer wall which often remains the lower flared fragments that not attached to the new conidiogenous cell, with up to three proliferations observed. Conidia acrogenous, clustered in masses, mostly globose to subglobose, rarely obovoid, 1-distoseptate, verruculose, pale brown or grayish green when young, olivaceous brown to mid brown when mature, 20–25 × 19–24 µm (\({\overline{\text{x}}}\) = 22.6 × 21.3 µm, n = 30), guttulate, thick-walled, truncate or concave at the base with a minute marginal frill. Sexual morph: Undetermined.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS62-1 (HKAS 112622, holotype; HKAS 125928, isotype).
Notes: Attempts to isolate this species were unsuccessful on WA, PDA and MEA media as the conidia did not germinate. DNA of the species was extracted from the fruiting bodies.
Uvarisporella resembles Exserticlava in macronematous, mononematous conidiophores, terminal, capitate, polyblastic conidiogenous cells and brown, distoseptate conidia that secede schizolytically (Hughes 1978; Tsui et al. 2001a). Uvarisporella aquatica is similar to Exserticlava globosa in globose to subglobose, brown, 1-distoseptate, verruculose conidia with comparable dimensions and overlapping in the length of conidiophores (Rao and de Hoog 1986). Conidiophores of Uvarisporella aquatica are shorter than that in the holotype of Exserticlava globosa (up to 400 µm) (Rao and de Hoog 1986), which was re-examined by Tsui et al. (2001a) as 120–180 µm long, but longer than other collections in Chang (1995) (up to 270 µm) and Tsui et al. (2001a) (90–180 µm). Conidia of Uvarisporella aquatica are larger than Exserticlava globosa’s holotype (17–21 µm diam.) and that in Tsui et al. (2001a) (16–25 × 16–20 µm) and almost identical to that in Chang (1995) (18–25 × 17–24 µm). Exserticlava globosa can be distinguished by percurrent proliferations of conidiogenous cells of which the old tip is not swollen and remains old outer wall appressed to the new conidiogenous cell like annellations and proliferating closely.
Among species in Exserticlava, molecular DNA data is only available for the type species E. vasiformis which was placed in Chaetosphaeriaceae and linked to the sexual morph Chaetosphaeria capitata (Fernández and Huhndorf 2005; Hyde et al. 2020a). The sequence of Uvarisporella aquatica was obtained from direct sequencing with only LSU gene amplified successfully. Molecular analysis revealed the placement of Uvarisporella aquatica within Tilachlidiaceae, Hypocreales which is distant to Exserticlava (Fig. 53). Exserticlava globosa and some other species in the genus are clearly differ from the generic type by the absence of hyaline upward conidiogenous extensions. Molecular evidence is therefore needed to resolve the systematic placement of Exserticlava and confirm the distinction between E. globosa and Uvarisporella aquatica.
Magnaporthales Thongk., Vijaykr. & K.D. Hyde
Notes: Thongkantha et al. (2009) introduced Magnaporthales with a single family Magnaporthaceae based on a phylogenetic analysis of LSU and SSU sequence data. Through an LSU-RPB1 phylogeny, Klaubauf et al. (2014) separated two new families, Pyriculariaceae and Ophioceraceae, from Magnaporthaceae. Later, Pseudohalonectriaceae and Ceratosphaeriaceae were introduced to the order by Hongsanan et al. (2017) and Luo et al. (2019) based on a combined dataset of LSU, SSU, TEF1α, and RPB2 sequences. Hyde et al. (2017a) have considered Distoseptisporaceae as a member of Magnaporthales based on a molecular clock analysis. However, Distoseptisporaceae was raised to the order level as Distoseptisporales (Luo et al. 2019). Hence, Magnaporthales contains five families Ceratosphaeriaceae, Magnaporthaceae, Ophioceraceae, Pseudohalonectriaceae, and Pyriculariaceae. Members of Magnaporthales are plant pathogens, endophytes, and saprobes, and most species have geographical, host, and tissue specificity (Feng et al. 2021).
Ceratosphaeriaceae Z.L. Luo, H.Y. Su & K.D. Hyde
Notes: Based on a single gene phylogeny of LSU and SSU sequences, respectively, Réblová (2006) considered Ceratosphaeria as a member of Magnaporthaceae. With a broader sampling and a multi-loci phylogeny, Luo et al. (2019) introduced Ceratosphaeriaceae to accommodate Ceratosphaeria.
Ceratosphaeria Niessl
Notes: Ceratosphaeria was established by Niessl (1876) with C. lampadophora (synonym: Sphaeria lampadophora) as the type species. Ceratosphaeria comprises 27 species with many described in the eighteenth and nineteenth centuries (Index Fungorum 2022). Molecular data is only available for the non-type strains of the generic type and a few recently described species. Ceratosphaeria is characterized by immersed to superficial, dark brown to black ascomata with a black or yellow neck, cylindrical, unitunicate asci with a non-amyloid apical ring, and narrowly cylindric-fusiform, or filiform ascospores (Luo et al. 2019). Many old Ceratosphaeria species, such as C. bicellula, C. caespitosa, C. mycophila and C. pusilla, need a re-evaluation of their taxonomic placement, because their short ellipsoidal to fusiform ascospores are distinct from the long cylindric-fusiform ascospores of the type species (Index Fungorum 2022).
Ceratosphaeria flava J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF559711, Facesoffungi number: FoF12825, Fig. 80
Etymology: referring to the yellow ascospores.
Holotype: MFLU 15-1171
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: conspicuous as yellow necks on the host surface and masses of pale pink asci at the apex of necks. Ascomata 200–300 × 300–500 μm, solitary or in small groups, immersed, perithecial, globose to subglobose, brown to yellowish brown, ostiolate, with a long yellow neck, ascospore masses pink to pale orange. Ostiole periphysate. Ascomatal wall coriaceous, 7–20 μm thick, 2-layered, outer layer consisting of multi-row of brown, polyhedral cells of textura angularis, outer row less distinguished from the host tissue; inner layer consisting of pale brown to hyaline, elongated, thin-walled cells of taxtura prismatic. Paraphyses abundant, persistent, septate, hyaline, unbranched, guttulate, tapering towards the apex, up to 200 µm long, (5–)8–12 μm wide near the base, slightly constricted at the septa. Asci (105–)120–140(–150) × (6–)8–12 µm (\({\overline{\text{x}}}\) = 130 × 9 µm, n = 30), cylindrical, straight, or slightly curved, obtuse at the apex, 8-spored, with a non-amyloid apical ring. Ascospores 100–120 × 1.5–3 µm (\({\overline{\text{x}}}\) = 110 × 2.3 µm, n = 30), parallel, filiform, straight or curved, hyaline, multi-septate, smooth-walled, guttulate, thin-walled.
Culture characteristics: Ascospores germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on MEA medium reaching 10–15 mm diam. after 2 weeks at 25 °C, in natural light, circular, with velvety, brown mycelium on the rough surface, sparser and dark brown at the edge; in reverse dark brown in the middle, paler at the entire margin.
Material examined: THAILAND, Chiang Rai, Tham Luang Nang Non Cave, on decaying wood submerged in a freshwater stream, 25 November 2014, J. Yang, YJ-5 (MFLU 15-1171, holotype; HKAS 112170, isotype), ex-type cultures MFLUCC 15-0058 and GZCC 16-0006.
Notes: Ceratosphaeria flava resembles C. aquatica, C. lignicola, C. phialidica, C. suthepensis, and C. yunnanensis in having immersed ascomata with a long yellowish neck, cylindrical to fusiform asci with a non-amyloid apical ring and hyaline filiform ascospores (Shearer 1989; Promputtha et al. 2004; Luo et al. 2019; Manawasinghe et al. 2022). However, they can be well distinguished by the dimensions of asci and ascospores (Table 4). Ascomata are immersed to superficial in C. phialidica with orange-brown amorphous material while they are immersed in the other five species. Asci and ascospores of C. flava are longer than that in C. aquatica, C. lignicola, C. phialidica, and C. yunnanensis, but shorter than C. suthepensis (Table 4). In the phylogenetic tree (Fig. 79), C. flava (MFLUCC 15-0058) clustered within Ceratosphaeria as a sister taxon to C. suthepensis (PDD 76762). The LSU sequence of C. flava differs from C. aquatica, C. lignicola, C. suthepensis, and C. yunnanensis by 10 bp (782/792 bp, one gap), 11 bp (802/813 bp), 15 bp (821/836 bp, one gap) and 9 bp (844/853 bp), respectively. The ITS sequence is not available for C. suthepensis. In the ITS gene region, C. flava differs from C. aquatica, C. lignicola, and C. yunnanensis by 24 bp (462/486 bp, eight gaps), 14 bp (471/485 bp, six gaps), and 28 bp (515/543 bp, four gaps), respectively. We therefore introduce C. flava as a new species based on the morphology and molecular evidence.
Maximum likelihood majority rule consensus tree for Magnaporthales using LSU, SSU, ITS, and TEF1α sequence data. Bootstrap support values for maximum likelihood (ML) greater than 75% and bayesian posterior probabilities greater than 0.95 are indicated near branches as ML BS/PP. The scale bar represents the expected number of changes per site. The tree is rooted with Myrmecridium sorbicola (CBS 143433) and Myrmecridium schulzeri (CBS 100.54). The new species are in blue and the new combination in black bold. Ex-type strains are marked with T after the strain number. Families are indicated in colored blocks. Branches with 100% ML BS and 1.0 PP are thickened
Ceratosphaeria flava (MFLU 15-1171, holotype) a Herbarium material. b Ascomata on wood. c Masses of asci at the apex of yellow neck protruding from the wood. d Section of an ascoma. e Section of peridium. f Paraphyses. g–j Asci. k–m Ascospores. n Apical ring. o Germinated ascospore. p, q Culture, p from above, q from below. Scale bars: b = 1000 μm, c = 200 μm, d = 100 μm, h, j = 40 μm, g, i, k–m, o = 30 μm, f = 20 μm, n = 15 μm, e = 10 μm
Ceratosphaeria suthepensis (I. Promputtha) J. Yang & K.D. Hyde, comb. nov.
Index Fungorum number: IF559712, Facesoffungi number: FoF12826
Basionym: Pseudohalonectria suthepensis I. Promputtha, Cryptog Mycol 25(1): 44 (2004)
Synonym: Tropohalonectria suthepensis (I. Promputtha) R.H. Perera, E.B.G. Jones & K.D. Hyde, Phytotaxa 278(2): 128 (2016)
Holotype: Thailand, Chiang Mai Province, Doi Suthep-Pui National Park, on dead leaves of Magnolia liifera, 31 July 2001, I. Promputtha, PDD 76762.
Notes: In the LSU-SSU-TEF1α phylogenetic analysis in Perera et al. (2016a), Pseudohalonectria suthepensis formed a basal branch within Magnaporthales and was distinct from Pseudohalonectria clade. Based on the molecular evidence and morphological differences, Perera et al. (2016a) introduced a new genus Tropohalonectria to accommodate P. suthepensis. Pseudohalonectria suthepensis was placed close to Ceratosphaeria lampadophora in Perera et al. (2016a). With a broader sampling of Ceratosphaeria taxa, our phylogenetic analysis showed that Pseudohalonectria suthepensis grouped within Ceratosphaeria as sister to C. flava (Fig. 79). Pseudohalonectria suthepensis resembles C. aquatica, C. flava, C. lignicola, and C. yunnanensis in having immersed ascomata with a long yellowish neck and outer layer of peridium less distinct from the host tissue, cylindrical asci with a non-amyloid apical ring and hyaline filiform ascospores (Luo et al. 2019; Manawasinghe et al. 2022; this study). We therefore recognize Pseudohalonectria suthepensis as a member of Ceratosphaeria.
Ophioceraceae Klaubauf, E.G. LeBrun & Crous
Notes: Ophioceraceae is a monotypic family typified by Ophioceras that was separated from Magnaporthaceae (Klaubauf et al. 2014). Taxa in Ophioceraceae are mainly saprobes growing on dead decaying wood, leaves, or herbaceous plants from aquatic or terrestrial habitats with a worldwide distribution (Shearer et al. 1999; Thongkantha et al. 2009; Klaubauf et al. 2014; Luo et al. 2019; Jiang et al. 2021b). Currently, Feng et al. (2021) found that several endophytic fungal strains isolated from healthy Poaceae samples belonged to Ophioceraceae based on a phylogenetic analysis using ITS, LSU, MCM7, RPB1, and TEF1α sequences.
Ophioceras Sacc.
Notes: Saccardo (1883) established Ophioceras with O. dolichostomum as the type species. The genus is characterized by black, immersed to superficial ascomata with a long neck, cylindrical to fusoid asci with a refractive apical ring, and hyaline filiform ascospores (Shearer et al.1999; Tsui et al. 2001b; Thongkantha et al. 2009; Klaubauf et al. 2014). Ceratosphaerella was found to cluster within Ophioceras clade in the multi-gene phylogen (Perera et al. 2016a; Luo et al. 2019; Jiang et al. 2021b). The genus was therefore synonymized to Ophioceras by Jiang et al. (2021b).
Ophioceras thailandense J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF559765, Facesoffungi number: FoF12827; Fig. 81
Ophioceras thailandense (MFLU 15–1138, holotype) a Herbarium material. b Ascomata on wood. c Section of an ascoma. d Section of peridium. e Paraphyses. f–i Asci. j–l Ascospores. n Apical ring. m Germinated ascospore. o, p Culture, o from above, p from below. Scale bars: b = 500 μm, c = 50 μm, m = 30 μm, e–l = 20 μm, d, n = 10 μm
Etymology: referring to the country Thailand where the species was collected.
Holotype: MFLU 15-1138.
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 100–350 μm high, 80–200 μm diam., solitary or in small groups, immersed or semi-immersed, perithecial, globose to subglobose, dark brown, ostiolate, with a long neck 1–1.5 mm. Ostiole periphysate. Ascomatal wall coriaceous, 9–24 μm thick, consisting of multi-layered, polyhedral cells of textura angularis, outer layers dark brown, becoming pale brown to hyaline at inner layers. Paraphyses numerous, persistent, septate, hyaline, unbranched, tapering towards the apex, 5–7 μm wide near the base, slightly constricted at the septa. Asci 80–110 × 7–11.5 µm (\({\overline{\text{x}}}\) = 95 × 9.7 µm, n = 25), cylindrical to fusiform, usually curved, obtuse at the apex, 8-spored, with a non-amyloid apical ring. Ascospores 75–91 × 2–3 µm (\({\overline{\text{x}}}\) = 83 × 2.6 µm, n = 20), parallel, filiform, straight to slightly curved, hyaline, multi-septate, smooth-walled, guttulate, thin-walled.
Culture characteristics: Ascospores germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on MEA medium reaching 10–15 mm diam. after 2 weeks at 25 °C, in natural light, circular, wrinkled, with dense white mycelium in the middle, sparser towards the edge; in reverse yellowish brown in the middle, pale yellow at the entire margin.
Material examined: THAILAND, Chiang Rai, Tham Luang Nang Non Cave, on decaying wood submerged in a freshwater stream, 25 November 2014, J. Yang, YJ-13 (MFLU 15-1138, holotype; HKAS 112173, isotype), ex-type cultures MFLUCC 15-0603 and GZCC 16-0007.
Notes: Ophioceras thailandense is similar to several Ophioceras species in having uniloculate ascomata with a long neck, cylindrical to fusoid asci with a non-amyloid apical ring, and hyaline, filiform, multi-septate ascospores, with an overlapping length of asci and ascospores (Table 5). However, Ophioceras thailandense is distinguished from these species by smaller ascomata (Table 5). Asci and ascospores of O. thailandense are larger than that in O. bambusae, O. indicus, O. leptosporum, and O. tenuisporum, smaller than O. hongkongense and O. sorghi, and within the dimension range of O. commune and O. fusiforme (Table 5). In the phylogenetic analysis, O. thailandense (MFLUCC 15-0603) formed a sister clade to O. commune (HKAS 92590, HKAS 92640 and BCC 3328) with strong statistical support (100 ML BS/1.0 PP) (Fig. 79). The ITS sequences of O. thailandense and O. commune revealed 95.33% (470/493 bp, five gaps) similarity. We therefore identify O. thailandense as a new species based on the morphology and molecular evidence (Jeewon and Hyde 2016).
Pseudohalonectriaceae Hongsanan & K.D. Hyde
Notes: Pseudohalonectriaceae was introduced by Hongsanan et al. (2017) based on the multi-gene phylogeny and molecular clock analysis. The family is monotypic with Pseudohalonectria as the type. Some Pseudohalonectria species can produce azaphilone compounds that have antagonistic activity against several pests, weeds, nematodes, bacteria, and fungi (Asthana and Shearer 1990; Foremska et al. 1992; Dong et al. 2004, 2006; Park et al. 2005).
Pseudohalonectria Minoura & T. Muroi
Notes: Minoura and Muroi (1978) established Pseudohalonectria based on P. lignicola which was isolated from submerged balsa wood in a lake in Japan. The genus is characterized by immersed to semi-immersed, bright yellow to brown ascomata with a long neck, unitunicate, cylindrical, clavate, or fusiform asci with a non-amyloid, thimble-shaped, refractive apical ring and cylindrical, fusiform, or filiform, hyaline to slightly pigmented, septate ascospores (Shearer 1989; Perera et al. 2016a; Luo et al. 2019). A phialidic asexual morph with hyaline, allantoid aseptate conidia was described for P. aomoriensis and P. phialidica (Shearer 1989; Ono and Kobayashi 2001). Huhndorf et al. (2008) later transferred P. phialidica to Ceratosphaeria. Currently, 14 species are accepted in Pseudohalonectria (Chethana et al. 2021; Index Fungorum 2022). The generic concept of Ceratosphaeria, Ophioceras, and Pseudohalonectria overlaps, however, they are well distinct by molecular data (Luo et al. 2019; Hyde et al. 2020a; Jiang et al. 2021b; Feng et al. 2021).
Pseudohalonectria aurantiaca J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF559766, Facesoffungi number: FoF12828; Fig. 82
Pseudohalonectria aurantiaca (MFLU 15-1174, holotype) a Herbarium material. b Colony on wood. c Ascoma on wood. d Section of an ascoma. e Section of peridium. f Paraphyses. g–l Asci. m–s Ascospores. t Germinated ascospore. u, v Culture, u from above, v from below. Scale bars: b = 500 μm, c = 100 μm, d = 60 μm, i–l, t = 40 μm, g, h = 30 μm, e, f, m, n, p–s = 20 μm, o = 15 μm
Etymology: referring to the orange masses of ascospores
Holotype: MFLU 15-1174
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: conspicuous as black necks on the host surface and masses of orange ascospores on the apex of necks. Ascomata 200–450 × 120–400 μm, scattered or aggregated, immersed, perithecial, subglobose to obpyriform, yellowish brown becoming dark brown, ostiolate, with a 300–530 μm long neck. Ostiole periphysate. Ascomatal wall coriaceous, 9–16.5 μm thick, outer layer yellowish brown, undifferentiated from plant tissue, inner layer consisting of hyaline, elongated, thin-walled cells of textura angularis or taxtura prismatic. Paraphyses abundant, persistent, septate, hyaline, unbranched, guttulate, tapering towards the apex, 6–8 μm wide near the base, strongly constricted at the septa. Asci 100–160 × 15–29 µm (\({\overline{\text{x}}}\) = 130 × 22 µm, n = 30), fusiform, straight or slightly curved, truncate at the apex, 8-spored, with a non-amyloid, thimble-shaped apical ring. Ascospores (35–)45–60(–70) × 6–9 µm (\({\overline{\text{x}}}\) = 52 × 7.8 µm, n = 40), overlapping, 2–3-seriate, cylindrical-fusiform, straight or slightly curved, hyaline when young, becoming pale yellow then pale orange when mature, 5-septate, smooth-walled, guttulate, thin-walled.
Culture characteristics: Ascospores germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on MEA medium slow growing, reaching 10–15 mm diam. after 1 month at 25 °C, in natural light, circular, with sparse, reddish-brown aerial mycelium on the surface; in reverse dark brown with entire margin.
Material examined: THAILAND, Chiang Rai, Tham Luang Nang Non Cave, on decaying wood submerged in a freshwater stream, 25 November 2014, J. Yang, YJ-12 (MFLU 15-1174, holotype; HKAS 112172, isotype), ex-type cultures MFLUCC 15-0379 and GZCC 15-0053.
Notes: Pseudohalonectria aurantiaca matches the generic circumscription of Pseudohalonectria. It resembles P. fagicola, P. hampshirensis, P. lignicola, and P. lutea in having immersed, somewhat yellowish ascomata becoming darkened, with a long neck, unitunicate asci with a thimble-shaped apical ring, and hyaline to pale orange or pale brown, cylindrical to cylindrical-fusiform ascospores (Minoura and Muroi 1978; Shearer 1989; Perera et al. 2016a). However, they differ by the dimensions of ascomata, asci and ascospores and septation (Table 6). Pseudohalonectria aurantiaca is distinguished by smaller ascomata and broader asci than the other four species (Table 6). Ascospores of P. aurantiaca are longer than that in P. fagicola, P. hampshirensis, shorter than P. lignicola, and with a similar length to P. lutea (Table 6). All these five species have hyaline to pigmented ascospores. However, ascospores of P. lutea are different by less pigmented end cells than the inner cells (Shearer 1989). In the phylogenetic analysis (Fig. 79), P. aurantiaca (MFLUCC 15-0379) formed a basal branch within Pseudohalonectria clade. Based on the morphology and molecular data, we recognize P. aurantiaca as a new species.
Myrmecridiales Crous
Notes: Myrmecridiales was introduced by Crous et al. (2015a) to accommodate Myrmecridium species. The order comprises a single family Myrmecridiaceae with two genera Myrmecridium and Neomyrmecridium (Hyde et al. 2020b).
Myrmecridiaceae Crous
Notes: Myrmecridiaceae is a monotypic family with Myrmecridium as the type genus (Crous et al. 2015a). Crous et al. (2018c) introduced the second genus Neomyrmecridium. Species in Myrmecridiaceae are reported as saprobes on leaf litter, stem, or leaves of herbaceous plants from terrestrial and aquatic habitats worldwide, with a few species occurring on soil and in house dust (Crous et al. 2015a, c, 2016, 2018a, c; Peintner et al. 2016; Réblová et al. 2016a; Tibpromma et al. 2017).
Myrmecridium Arzanlou, W. Gams & Crous
Notes: Myrmecridium was established by Arzanlou et al. (2007) to accommodate two ramichloridium-like taxa. Species of the genus are cosmopolitan and distributed in many countries, such as Australia, China, France, Germany, Ibid, Ireland, Korea, Netherlands, Spain, Thailand, Ukraine and Uruguay. (Arzanlou et al. 2007; Crous et al. 2011, 2012, 2013, 2015a, c, 2018a, c, 2020b, 2021; Jie et al. 2013; Peintner et al. 2016; Luo et al. 2019). They are commonly reported from soils and plant tissues (Crous et al. 2011; Jie et al. 2013; Peintner et al. 2016). Myrmecridium is a well-studied genus with 20 species and two varieties (Chethana et al. 2021; Index Fungorum 2022). Sexual morph is only known for M. montsegurinum (Réblová et al. 2016a). Sequence data are available for all the species in the genus.
Myrmecridium splendidum L.L. Liu, J. Yang, K.D. Hyde & Z.Y. Liu, sp. nov.
Index Fungorum number: IF559767; Facesoffungi number: FoF12829; Fig. 83
Etymology: referring to the glistening colony on nature substrates.
Holotype: GZAAS 20-0444
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on natural substrates effuse, scattered, or aggregated, hairy, brown, glistening. Mycelium mostly immersed, composed of septate, smooth-walled, pale brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, solitary or in small groups, lower part straight, upper part flexuous, cylindrical, reddish brown, slightly paler towards the apex, septate, unbranched, 125–270 × 3–6 μm (\({\overline{\text{x}}} \) = 170 × 4.5 μm, n = 15). Conidiogenous cells polyblastic, integrated, terminal and intercalary, determinate, sympodial, denticulate, flexuous, cylindrical, pale reddish brown to subhyaline. Conidia acropleurogenous, solitary, fusiform or narrowly obovoid, subhyaline to pale brown, 2–3-septate, finely verrucose, 14–24 × 4–5.5 μm (\({\overline{\text{x}}} \) = 18.5 × 5 μm, n = 30), arranged sympodially on the 3/4 of conidiophores. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinating on WA medium within 24 h. Colonies on PDA medium reaching about 20 mm diam. after 1 month at 25 °C in dark, circular, consisting of a matted felt with powdery appearance, with velvety white mycelium in the middle, sparse, yellowish in the inner ring and white in the outer ring; in reverse dark yellow in the middle, paler to white towards the entire margin.
Material examined: CHINA, Guizhou Province, Bijie City, Weining, Caohai national nature reserve, 26.817° N, 104.217° E, on decaying submerged aquatic plants in Caohai Lake, October 2018, L.L. Liu, 18C-64, (GZAAS 20-0444, holotype), ex-type culture GZCC 19-0549.
Notes: Myrmecridium splendidum resembles Neomyrmecridium aquaticum (synonym: Myrmecridium aquaticum) in having brown, relatively long conidiophores and obovoid, subhyaline to pale brown, 3-septate conidia (Luo et al. 2019). However, Myrmecridium splendidum has shorter conidiophores (125–270 μm vs. 211–308 μm) and longer conidia (14–24 μm vs. 14–16 μm) than that of Neomyrmecridium aquaticum (Luo et al. 2019). In the phylogenetic analysis, M. splendidum (GZCC 19-0549) clustered with M. montsegurinum (PRM 934684) and M. hiemale (CBS 141017) within Myrmecridium clade (Fig. 55). It is unable to compare the morphology between M. montsegurinum and M. splendidum as the asexual morph is unknown to the former. However, they are phylogenetically distinct. Based on the molecular data, M. splendidum differs from M. montsegurinum by 26 base pairs (824/850 bp) of the LSU sequences and 51 base pairs (395/446 bp, 11 gaps) of the ITS sequences. Myrmecridium splendidum is similar to M. hiemale in having reddish brown conidiophores and obovoid to fusiform conidia (Peintner et al. 2016). However, M. splendidum differs from M. hiemale in having longer conidiophores (125–270 µm vs. up to 80 µm) and larger conidia (14–24 × 4–5.5 μm vs. 5.4–10.9 × 1.8–4 μm) (Peintner et al. 2016). In addition, conidia of M. splendidum are subhyaline to pale brown and 2–3-septate without a sheath while that of M. hiemale are hyaline and uniseptate with a wing-like gelatinous sheath (Peintner et al. 2016). The LSU and ITS sequences of M. splendidum and M. hiemale showed 31 bp (504/535 bp) and 59 bp (426/485 bp, 15 gaps) differences, respectively.
Neomyrmecridium Crous
Notes: Neomyrmecridium was established by Crous et al. (2018c) to accommodate three species Neomyrmecridium septatum, N. asiaticum and N. sorbicola based on morphological characters and phylogenetic analyses. Recently, two new species Neomyrmecridium asymmetricum and N. guizhouense are introduced in the genus (Hyde et al. 2020b; Serrano et al. 2020). Myrmecridium aquaticum was transferred to Neomyrmecridium and synonymized under N. aquaticum (Crous et al. 2021). Currently, with available sequence data, six species are accepted in Neomyrmecridium (Chethana et al. 2021; Index Fungorum 2022).
Neomyrmecridium is characterized by solitary, unbranched conidiophores, polyblastic, denticulate conidiogenous cells, and fusoid-ellipsoid, septate conidia with upper two-thirds encased in a mucoid sheath (Crous et al. 2018a, c; Hyde et al. 2020b). Species in the genera are reported from freshwater and terrestrial habitats in China, Ecuador, Thailand and Germany (Crous et al. 2018a, c; Hyde et al. 2020b; Serrano et al. 2020). In this study, we introduce a new freshwater species Neomyrmecridium naviculare based on the morphology and phylogeny.
Neomyrmecridium naviculare J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559768; Facesoffungi number: FoF12830; Fig. 84
Etymology: referring to the navicular conidia.
Holotype: HKAS 124639
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, scattered or aggregated, dark brown, glistening. Mycelium mostly immersed, composed of septate, smooth, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, lower part straight, upper part flexuous, cylindrical, smooth-walled, septate, unbranched, brown, pale brown to subhyaline at the upper part, 100–200 × 4–5.6 µm (\({\overline{\text{x}}}\) = 158 × 4.8 µm, n = 15). Conidiogenous cells polyblastic, integrated, terminal, becoming intercalary, determinate, sympodial, denticulate, flexuous, brown to subhyaline. Conidia acropleurogenous, navicular to fusiform, tapering to a hilum towards the base, obtuse at the apex, (1–)3–septate, verrucose, hyaline when young, pale brown when mature, paler at both ends, 16–24 × 5.5–7.5 µm (\({\overline{\text{x}}}\) = 19.6 × 6.5 µm, n = 45), guttulate, thin-walled, with a thin mucilaginous sheath at the middle part. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm diam. after 1 month at 25 °C in dark, circular, producing yellow pigment, wrinkled, with velvety, yellowish white mycelium on the surface; in reverse dark yellow with entire margin.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying wood submerged in Suoluo River, 17 October 2018, J. Yang, GDT38-1 (HKAS 124639, holotype), ex-type culture GZCC 20-0484; ibid, GDT41-1 (HKAS 112640, paratype), ex-paratype culture MFLUCC 19-0303.
Notes: In the phylogenetic analysis (Fig. 55), Neomyrmecridium naviculare (GZCC 20-0484 and MFLUCC 19-0303) clustered as a sister taxon to N. septatum (CBS 145073). Neomyrmecridium naviculare resembles N. septatum in having solitary, septate, unbranched conidiophores, polyblastic, terminal, denticulate conidiogenous cells and solitary, hyaline to pale brown, 3-septate conidia with a mucilaginous sheath (Crous et al. 2018c). However, Neomyrmecridium naviculare possesses navicular to fusiform conidia that are larger than the fusoid-ellipsoid conidia of N. septatum (16–24 × 5.5–7.5 µm vs. 12–20 × 3.5–5 µm) (Crous et al. 2018c). Conidiophores of N. naviculare are mid-brown and longer than the subhyaline to pale brown of N. septatum (100–200 µm vs. 40–70 µm) (Crous et al. 2018c). Comparisons of the LSU, ITS, and RPB2 sequences of N. naviculare and N. septatum revealed 99.32% (872/878 bp), 92.19% (484/525 bp, 15 gaps) and 88.65% (734/828 bp) sequence similarity, respectively.
Pleurotheciales Réblová & Seifert
Notes: Pleurotheciales was introduced at the ordinal rank based on the phylogenetic analyses of combined ITS, SSU, LSU, β-tubulin, mcm7, and RPB2 sequences (Réblová et al. 2016c). The order is monotypic with the type family Pleurotheciaceae which accommodates twelve genera (Hyde et al. 2020a; Réblová et al. 2020a; Dong et al. 2021b). Hyde et al. (2020a) and Réblová et al. (2020a) illustrated the changed history of the problematic taxa in the order. Xia et al. (2017) provided reference sequences of two Rhexoacrodictys species considered in Savoryellaceae. With further phylogenetic analysis, Luo et al. (2019) accepted these two Rhexoacrodictys species in Pleurotheciaceae which was ignored by Hyde et al. (2020a). The phylogenetic result in this study (Fig. 53) agrees with Luo et al. (2019) to treat Rhexoacrodictys as a member of Pleurotheciaceae.
Pleurotheciaceae Réblová & Seifert
Notes: Pleurotheciaceae includes five holomorphic genera, Helicoascotaiwania (Fallah et al. 1999; Réblová et al. 2020a), Melanotrigonum (Réblová et al. 2016c), Pleurothecium (Fernández et al. 1999), Pleurotheciella (Réblová et al. 2012) and Sterigmatobotrys (Réblová and Seifert 2011). Adelosphaeria is known in sexual morph with brown, subglobose, aseptate cells without forming a typical asexual morph (Réblová et al. 2016c). These sexual morphs of Pleurotheciaceae are similar in nondescript morphology, and only Helicoascotaiwania is clearly distinguished from the others by versicolor ascospores. Réblová et al. (2016c) compared these sexual morphs with Aquaticola, Annulatascus, Annulusmagnus and Chaetosphaeria. Asexual morphs in Pleurotheciaceae possess macronematous or semi-macronematous, mononematous or synnematous conidiophores, monoblastic or polyblastic or thallic conidiogenous cells, sometimes elongating percurrently and hyaline or brown or versicolor, aseptate or septate even muriform conidia, conidial secession rhexolytic or schizolytic (Dong et al. 2021b). Identification of Pleurotheciaceae taxa is based on growth in culture and their asexual morphs with molecular data support.
Dematipyriforma L.Y. Sun, Hai-Yan Li, Xiang Sun & L.D. Guo
Notes: Dematipyriforma is a monotypic genus with D. aquilariae as the type species (Sun et al. 2017). Dematipyriforma aquilariae is an endophyte isolated from the trunk of Aquilaria crassna in Laos. It produces trichocladium-like chlamydospores and canalisporium-like conidia in culture.
Dematipyriforma aquilariae L. Y. Sun, Hai-Yan Li, Xiang Sun & L.D. Guo, Cryptog Mycol 38(3): 345 (2017)
Index Fungorum number: IF842402; Facesoffungi number: FoF12831; Fig. 85
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, scattered or aggregated, black, glistening, conidia arising from aerial hyphae. Aerial hyphae smooth, septate, sparsely branched, hyaline to pale brown, thin-walled. Conidiophores reduced to conidiogenous cells. Conidiogenous cells monoblastic, integrated, cylindrical to ellipsoidal, brown. Conidia acrogenous, subglobose, ellipsoidal or pyriform, muriform when young with 3 transverse septa and 2 longitudinal septa, darkened without visible septa when mature, smooth-walled, dark brown, with a slightly paler, papillate basal cell, 28–44 × 22–32 µm (\({\overline{\text{x}}}\) = 34 × 25.5 µm, n = 30). Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h and germ tubes produced from the base. Colonies on MEA medium slow growing, reaching 10–15 mm in 1 month at 25 °C, in natural light, circular, with velvety, dark grayish green mycelium on the surface; in reverse dark green to black with entire margin.
Material examined: THAILAND, Rayong Province, Klaeng, on decaying wood submerged in a freshwater stream, 24 April 2017, Y.Z. Lu, RAY-11 (MFLU 22-0076 and HKAS 112643), living cultures MFLUCC 17-2382 and GZCC20-0416.
Notes: In the phylogenetic analysis (Fig. 53), our collection clustered as sister taxon to the type material of Dematipyriforma aquilariae (CGMCC 3.17268). Molecular data of protein-coding gene is unavailable for the type material. The LSU, ITS, and SSU sequences are available for both isolates and their sequence similarity was revealed to be 100% (618/618 bp), 99.27% (545/549, two gaps) and 99.90% (1008/1009 bp), respectively. Our collection resembles the holotype of D. aquilariae in having muriform conidia arising from hyphae with conidiophores reduced to conidiogenous cells. Conidia of our collection are subglobose, ellipsoidal to pyriform, dark brown and larger than that in the holotype [28–44 × 22–32 µm (\({\overline{\text{x}}}\) = 34 × 25.5 µm) vs. 25–37.5 × 15–22.5 µm (\({\overline{\text{x}}}\) = 32.9 × 18.8 µm)] which are pyriform or elongated pyriform and pale brown. Based on the molecular evidence, we identify our collection as D. aquilariae, describe the species as a saprobe on natural substrate and report it as a new record in Thailand.
Pseudodactylariales Crous
Notes: Pseudodactylariales was introduced by Crous et al. (2017) comprising one family with a single genus Pseudodactylaria. Pseudodactylariales was placed in Sordariomycetidae based on the phylogenetic analyses and divergence time estimates (Hyde et al. 2020a).
Pseudodactylariaceae Crous
Notes: Pseudodactylariaceae is a well-studied family introduced by Crous et al. (2017) and typified by Pseudodactylaria. Species in the family were reported as saprobes from freshwater and terrestrial habitats in Australia, China, and Thailand (Tsui et al. 1997; Crous et al. 2017; Lin et al. 2018; Hyde et al. 2020b; Lu et al. 2020; Bao et al. 2021b; Boonmee et al. 2021).
Pseudodactylaria Crous
Notes: Pseudodactylaria was established for two dactylaria-like species, P. hyalotunicata and P. xanthorrhoeae (Crous et al. 2017). Currently, seven species are accepted in the genus (Chethana et al. 2021; Index Fungorum 2022). Pseudodactylaria is characterized by single, unbranched, septate conidiophores, polyblastic, denticulate conidiogenous cells and solitary, fusoid-ellipsoid, hyaline conidia (Lin et al. 2018; Hyde et al. 2020b; Lu et al. 2020; Bao et al. 2021b). In this study, we introduce three new species, P. denticulata, P. longidenticulata and P. uniseptata based on the phylogeny and morphology.
Pseudodactylaria denticulata J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF559822; Facesoffungi number: FoF12833; Fig. 86
Etymology: referring to the denticulate conidiogenous cells.
Holotype: MFLU 22-0068
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, scattered or aggregated, brown, with glistening conidial masses at apex. Mycelium partly superficial, partly immersed, composed of septate, smooth-walled, pale brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight or slightly flexuous, cylindrical, smooth-walled, septate, unbranched, brown, paler towards the apex, thick-walled, 70–235 × 3–5.5 µm (\({\overline{\text{x}}}\) = 135 × 4 µm, n = 25). Conidiogenous cells polyblastic, integrated, terminal, cylindrical, pale brown to subhyaline, 7–18 µm long, 3–4 µm wide, denticulate, with up to 10 denticles at the apex. Conidia acrogenous, narrowly fusiform or cylindrical, hyaline, uniseptate, smooth-walled, guttulate, 21–28 × 2–3.5 µm (\({\overline{\text{x}}}\) = 24 × 3 µm, n = 25), thin-walled, sometimes with an inconspicuous hyaline appendage. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on MEA medium slow growing, reaching 5–10 mm diam. after 2 months at 25 °C in natural light, with dense mycelium on the surface, grayish brown in the middle, white in the inner ring and brown in the outer ring; in reverse brown in the middle and paler at the entire margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT18-2 (MFLU 22-0068, holotype; HKAS 112154, isotype), ex-type cultures MFLUCC 17-2125 and GZCC 20-0390.
Notes: Pseudodactylaria denticulata resembles P. aquatica, P. camporesiana, P. longidenticulata and P. uniseptata in having brown conidiophores, polyblastic denticulate conidiogenous cells and hyaline, narrowly fusiform, uniseptate conidia (Hyde et al. 2020b; Bao et al. 2021b; this study). The other species in the genus, P. albicolonia, P. brevis, P. fusiformis P. hyalotunicata and P. xanthorrhoeae are distinct from the above species by hyaline conidiophores (Tusi et al. 1997; Crous et al. 2017; Lin et al. 2018; Lu et al. 2020; Boonmee et al. 2021). Pseudodactylaria denticulata is distinguished from P. aquatica, P. camporesiana, P. longidenticulata and P. uniseptata by longer conidiophores, shorter conidiogenous cells, and longer but slightly narrower conidia (Table 7).
The phylogenetic analysis showed that Pseudodactylaria denticulata (MFLUCC 17-2125) clustered as a sister taxon to P. aquatica (MFLUCC 18-0201) with strong statistical support (100% ML BS/1.0 PP) (Fig. 53). Comparison of the ITS gene region revealed 20 bp (506/526 bp, six gaps) differences between P. denticulata and P. aquatica. We therefore recognize P. denticulata as a new species following the guidelines of Jeewon and Hyde (2016).
Pseudodactylaria longidenticulata J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF559823; Facesoffungi number: FoF12834; Fig. 87
Pseudodactylaria longidenticulata (MFLU 22-0075, holotype) a, b Colony on wood. c–f Conidiophores. g Conidiogenous cell. h, i Conidiogenous cells with conidia. j–p Conidia, arrows indicate appendages. q Germinated conidium. r, s Culture, r from above, s from below. Scale bars: a, b = 100 µm, c–f = 30 µm, g–j, q = 20 µm, k–p = 15 µm
Etymology: referring to the long and denticulate conidiogenous cells.
Holotype: MFLU 22-0075
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, scattered or aggregated, brown, with glistening conidial masses. Mycelium mostly immersed, composed of septate, smooth-walled, pale brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight or flexuous, cylindrical, smooth-walled, septate, unbranched, brown, paler to hyaline towards the apex, thick-walled, (55–)80–130(–175) × 3–5 µm (\({\overline{\text{x}}}\) = 117 × 3.8 µm, n = 20). Conidiogenous cells polyblastic, integrated, terminal, indeterminate, cylindrical, denticulate, hyaline, 20–145 × 2.5–5 µm. Conidia acropleurogenous, narrowly fusiform, hyaline, uniseptate, smooth-walled, guttulate, 18–27 × 3–4.5 µm (\({\overline{\text{x}}}\) = 24 × 4 µm, n = 30), thin-walled, usually with a hyaline irregular appendage. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in natural light, circular, with dense mycelium on the surface, dark brown in the middle, white at the edge; in reverse dark brown in the middle and white at the entire margin.
Material examined: THAILAND, Rayong Province, Klaeng, on decaying wood submerged in a freshwater stream, 24 April 2017, Y.Z. Lu, RAY-4 (MFLU 22-0075, holotype; HKAS 112644, isotype), ex-type cultures MFLUCC 17-2383 and GZCC 20-0411.
Notes: Pseudodactylaria longidenticulata is distinguished from other species in the genus by longer conidiogenous cells (Table 7). In the phylogenetic tree (Fig. 53), Pseudodactylaria longidenticulata was positioned as a sister taxon to P. uniseptata. They differ by the dimensions of conidiophores and conidiogenous cells (Table 7). Comparisons of the LSU, ITS, TEF1α, and RPB2 gene regions of P. longidenticulata and P. uniseptata showed five bp (876/881 bp, one gap), 20 bp (501/52 1 bp, two gaps), 37 bp (893/930 bp) and 68 bp (972/1040 bp) differences, respectively. Therefore, Pseudodactylaria longidenticulata is introduced as a new species based on the morphology and molecular DNA data.
Pseudodactylaria uniseptata J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF559824; Facesoffungi number: FoF12835; Fig. 88
Etymology: referring to the uniseptate conidia
Holotype: MFLU 22-0072
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, scattered or aggregated, brown, with glistening conidial masses at apex. Mycelium mostly immersed, composed of septate, smooth-walled, pale brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight or slightly flexuous, cylindrical, smooth-walled, septate, unbranched, dark brown, paler to hyaline towards the apex, thick-walled, 90–185 × 3–5 µm (\({\overline{\text{x}}}\) = 140 × 3.7 µm, n = 20). Conidiogenous cells polyblastic, integrated, rarely discrete, terminal, cylindrical, denticulate, hyaline, 27–50 µm long about 1/3 of the conidiophore, 2.5–4 µm wide. Conidia acrogenous, narrowly fusiform, hyaline, uniseptate, smooth-walled, guttulate, 19–25 × 2.5–4 µm (\({\overline{\text{x}}}\) = 22 × 3.3 µm, n = 40), thin-walled, usually with a hyaline irregular appendage. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in natural light, with dense mycelium on the surface, pale brown in the middle, white in the inner ring and pale brown in the outer ring; in reverse brown in the middle and paler at the entire margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT29-2 (MFLU 22-0072, holotype; HKAS 112161, isotype), ex-type cultures MFLUCC 17-2395 and GZCC 20-0404.
Notes: Among Pseudodactylaria species, three new species, P. denticulata, P. longidenticulata and P. uniseptata, possess longer conidiophores (maximum length more than 100 µm) than that of the others (less than 100 µm) (Table 7). Pseudodactylaria denticulata, P. longidenticulata and P. uniseptata share similar morphology in having brown conidiophores, denticulate conidiogenous cells and hyaline, narrowly fusiform, uniseptate conidia usually with a hyaline irregular appendage. However, they can be distinguished by the dimensions of conidiophores, conidiogenous cells and conidia (Table 7). In the phylogenetic analysis (Fig. 53), Pseudodactylaria uniseptate was positioned as a sister taxon to P. longidenticulata.
Rhamphoriales K.D. Hyde & Hongsanan
Notes: Based on phylogeny and divergence time estimation, a robust clade of Rhamphoriaceae was raised to a systematic ordinal rank as Rhamphoriales (Hyde et al. 2021a). This monotypic order comprises four holomorphic genera, Rhamphoria, Rhamphoriopsis, Rhodoveronaea and Xylolentia, and an uncertain Linkosia species.
Rhamphoriaceae Réblová
Notes: Rhamphoriaceae was established to accommodate the above taxa with strong phylogenetic support using a combined LSU, SSU, and RPB2 dataset (Réblová and Štěpánek 2018). The family is characterized by perithecial, immersed or superficial ascomata with papillate, rostrate or long cylindrical neck, leathery to fragile, 2-layered peridium, non-amyloid apical annulus of asci and hyaline or brown, dictyoseptate or transversely septate ascospores that may produce ascoconidia within the asci (Müller and Samuels 1982a; Réblová and Štěpánek 2018; Hyde et al. 2020c). Asexual morphs in the family possess mononematous or loosely fasciculate, macronematous conidiophores or that reduced to conidiogenous cells, monoblastic or polyblastic conidiogenous cells and hyaline or brown, aseptate or septate conidia (Réblová and Štěpánek 2018; Hyde et al. 2020a, c). Members in Rhamphoriaceae mainly grow on decaying wood from terrestrial environments, rarely on a fungal colony or from freshwater habitats, reported predominantly from Europe, and some from Argentina, Australia and China (Arzanlou et al. 2007; Réblová and Štěpánek 2018; Luo et al. 2019; Hyde et al. 2020c; Yuan et al. 2020).
Rhamphoriopsis Réblová & Gardiennet
Notes: Rhamphoriopsis was introduced by Réblová and Štěpánek (2018) with a single species R. muriformis. Ascomata of R. muriformis grew on decaying twigs of Buxus sempervivens and were associated with phaeoisaria-like conidiophores. The life history of R. muriformis was established in vivo and in vitro. Later, the second species R. sympodialis was described in asexual state (Hyde et al. 2020c).
Rhamphoriopsis aquimicrospora J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559825; Facesoffungi number: FoF12836; Fig. 89
Etymology: referring to the aquatic habitat of the species and the small conidia.
Holotype: HKAS 124633
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, scattered or in small groups, hairy, visible as solitary, brown conidiophores with white conidial masses at the apex. Mycelium partly superficial, partly immersed, composed of septate, smooth-walled, pale brown to hyaline hyphae. Conidiophores macronematous, synnematous, erect, smooth-walled, septate, cylindrical, reddish brown, paler towards the apex, 100–180 µm long, 1.2–2.5 µm wide, compact parallelly forming synnemata which are 135–178 µm long, 5–11 µm wide at middle part, 9–20 µm wide near the base, with terminal of conidiophores splaying out as a flared head. Conidiogenous cells polyblastic, integrated, terminal, pale brown near the base, becoming hyaline towards the apex, smooth-walled, sympodially extending with numerous denticles. Conidia ellipsoidal, aseptate, smooth-walled, hyaline, 2–3.5 × 1.3–2 µm (\({\overline{\text{x}}}\) = 2.6 × 1.7 µm, n = 50). Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Colonies on PDA medium slow growing, reaching 5–10 mm diam. after 2 months at 25 °C in dark, circular, raised, waxy-mucoid, with sparse, finely floccose, light yellow aerial mycelium on the surface; in reverse yellow with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS43-2 (HKAS 124633, holotype), ex-type culture GZCC 20-0515.
Notes: Rhamphoriopsis aquimicrospora is an asexual morph without a known sexual morph. It resembles R. muriformis and R. sympodialis in having hyaline, polyblastic conidiogenous cells sympodially extending with numerous denticles and ellipsoidal, hyaline, aseptate, relatively small conidia (Réblová and Štěpánek 2018; Hyde et al. 2020c). However, R. aquimicrospora is distinguished by synnematous conidiophores and the dimensions of conidiophores and conidia. In the phylogenetic analysis (Fig. 53), R. aquimicrospora (GZCC 20-0515) formed a basal branch in Rhamphoriopsis clade.
Rhamphoriopsis is similar to Phaeoisaria which was positioned in Pleurotheciaceae. They share macronematous, mononematous or synnematous conidiophores, polyblastic conidiogenous cells with inconspicuous sympodial denticles and hyaline, ellipsoidal to obovoid conidia. Conidiophores of R. aquimicrospora splay out at the apical part of the synnemata, while those in Phaeoisaria often form tree-like synnemata, splaying out from the middle part. Rhamphoriopsis species produce smaller conidia than Phaeoisaria. An ophioceras-like sexual morph was linked to Phaeoisaria based on molecular evidence (Luo et al. 2019). Its filiform ascospores differ from the muriform ascospores of Rhamphoriopsis (Réblová and Štěpánek 2018).
Rhamphoriopsis glauca (Ellis & Everh.) J. Yang, Jian K. Liu & K.D. Hyde, comb. nov.
Index Fungorum number: IF559826; Facesoffungi number: FoF12837
Basionym: Chloridium glaucum Ellis & Everh., J Mycol 4 (11): 113 (1888)
Synonym: Phaeoisaria glauca (Ellis & Everh.) de Hoog & Papendorf, Persoonia 8 (4): 413 (1976)
Holotype: USA, Newfield, Gloucester County, on rotten wood of Quercus sp. July 1888, in herb. NY.
Notes: In our phylogenetic analysis (Fig. 53), Phaeoisaria glauca (CBS 480.75) (as Rhamphoriopsis glauca) nested within Rhamphoriopsis clade. Phaeoisaria glauca was transferred from Chloridium and described in vitro with conidiophores profusely branched and forming tufts, occasionally becoming synnema-like (de Hoog and Papendorf 1976). It possesses polyblastic, sympodial, denticulate conidiogenous cells and small, hyaline, aseptate, and guttuliform to ellipsoidal conidia (de Hoog and Papendorf 1976; Whitton et al. 2012). Phaeoisaria glauca is distinguished from the well-defined synnematous species of Phaeoisaria (de Hoog and Papendorf 1976; Liu et al. 2015a; Hyde et al. 2018; Luo et al. 2018a) and differs from the mononematous, unbranched conidiophores of P. fasciculata (Réblová et al. 2016c). However, conidiophores of Phaeoisaria glauca resemble Rhamphoriopsis muriformis in vitro. Based on the molecular DNA data and morphological characters, we recognize Phaeoisaria glauca as a member of Rhamphoriopsis. However, molecular data from the type material, discovery of sexual morph and other specimens of Phaeoisaria glauca (as Rhamphoriopsis glauca) are needed to confirm its systematic placement.
Rhodoveronaea Arzanlou, W. Gams & Crous
Notes: Based on the phylogenetic analysis inferred from ITS and LSU sequences, Arzanlou et al. (2007) established Rhodoveronaea to accommodate a veronaea-like species R. varioseptata, which was described in vitro. Réblová (2009) described the sexual morph of R. varioseptata with non-stromatic, immersed, dark ascomata with stout conical emerging neck, cylindrical long-stipitate asci with non-amyloid apical annulus and fusiform, septate, hyaline ascospores. The fertile conidiophores of R. varioseptata were associated with the perithecia on natural substrates. Their congeneric relationship was proved experimentally and phylogenetically (Réblová 2009). Rhodoveronaea varioseptata occurred on a fungal colony of Bertia moriformis, decaying wood, ectomycorrhizal root, and soil, predominantly from Europe (Czech Republic, Germany, Poland, and Sweden) and Thailand (Arzanlou et al. 2007; Réblová 2009; Phosri et al. 2012; Behnke-Borowczyk et al. 2020). The second species Rhodoveronaea aquatica is an asexual morph from decaying submerged wood in China (Luo et al. 2019).
Rhodoveronaea aquatica Z.L. Luo, K.D. Hyde & H.Y. Su, Fungal Divers 99: 531 (2019)
Index Fungorum number: IF555661, Facesoffungi number: FoF05435; Fig. 90
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, scattered or aggregated, brown, with glistening conidial masses at apex. Mycelium partly superficial, partly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, often flexuous at the conidogenous region, solitary or aggregated in small groups, cylindrical, smooth-walled, septate, unbranched, reddish brown, or mid brown, paler towards the apex, 70–147 × 3–4.5 µm (\({\overline{\text{x}}}\) = 118 × 3.7 µm, n = 20). Conidiogenous cells polyblastic, integrated, terminal, determinate, sympodial, flexuous, pale brown to subhyaline, pigmented, with inconspicuous denticles. Conidia acropleurogenous, aggregated in slimy masses, ellipsoidal to narrowly obovoid, 1–3-septate, mostly 3-septate, smooth-walled, pale yellowish brown, (8.5–)10–13 × 4–6 µm (\({\overline{\text{x}}}\) = 11.7 × 4.7 µm, n = 30), guttulate, thin-walled, sometimes slightly constricted at the septa. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium slow growing, reaching 10–15 mm diam. after 1 month at 25 °C in dark, with compact mycelium on the surface, pale yellow in the middle, yellowish brown at the edge; in reverse yellow in the middle, brown at the entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 6 July 2018, J. Yang, SGT16-2 (HKAS 112574), living cultures MFLUCC 19-0267 and GZCC 20-0447.
Notes: Our collection (GZCC 20-0447) grouped with Rhodoveronaea aquatica (MFLUCC 18-1339) as a sister taxon with strong support (100% MLBS/1.0 PP) (Fig. 53). Comparisons of the LSU, ITS, and TEF1α sequences of our specimen and the ex-type strain of R. aquatica revealed 99.75%, 98.8% and 98.4% similarity, respectively. RPB2 sequence is unavailable for the ex-type strain. We re-examined the holotype of R. aquatica and emended the dimensions as conidiophores 69–159 × 4.5–5.5 µm (\({\overline{\text{x}}}\) = 114 × 5 µm, n = 10) and conidia (6–)10.5–14.5 × 4.5–5.5 µm (\({\overline{\text{x}}}\) = 12.5 × 5 µm, n = 30). Thus, our collection well matches the morphology and emended dimensions of R. aquatica (Luo et al. 2019). Following the guidelines of Jeewon and Hyde (2016), we recognize our specimen as Rhodoveronaea aquatica.
Xylolentia Réblová
Notes: Xylolentia was introduced by Réblová and Štěpánek (2018) to accommodate a holomorphic species X. brunneola. The second species X. reniformis was described as an asexual morph (Yuan et al. 2020). These two Xylolentia species were isolated from decaying wood in the Czech Republic and China, respectively. Xylolentia is distinct from other genera in Rhamphoriaceae by uniseptate brown ascospores (Réblová and Štěpánek 2018).
Xylolentia aseptata J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559827; Facesoffungi number: FoF12838; Fig. 91
Etymology: referring to the aseptate conidia.
Holotype: HKAS 112647
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, scattered or aggregated, brown, with glistening conidial masses at apex. Mycelium partly superficial, partly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, solitary or aggregated in small groups, cylindrical, smooth-walled, septate, unbranched, mid to dark brown, becoming pale brown to subhyaline at the apex, 95–215 × 3.5–7 µm (\({\overline{\text{x}}}\) = 137 × 5 µm, n = 20). Conidiogenous cells polyblastic, integrated, elongating percurrently, terminal, determinate, cylindrical to cylindrical-lageniform, pale brown near the base, subhyaline to hyaline towards the apex, 18–26 × 3–5 µm, sympodially extending. Conidia acrogenous, aggregated in slimy masses, ellipsoidal to reniform, aseptate, smooth-walled, hyaline, 3–5.5 × 1.8–3 µm (\({\overline{\text{x}}}\) = 4 × 2.3 µm, n = 50), guttulate, thin-walled. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h. Colonies on PDA medium reaching 10–15 mm diam. in 2 weeks at 25 °C in dark, circular, raised, with dark brown mycelium on the plicated and waxy-mucoid surface; reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Dushan District, 25.965° N, 107.65° E, on decaying wood submerged in a freshwater stream, 26 Aug 2017, J. Yang, SG29-1 (HKAS 112647, holotype; HKAS 125925, isotype), ex-type cultures MFLUCC 18-0425 and GZCC 20-0424; ibid, SG37-2 (HKAS 124636, paratype), ex-paratype cultures MFLUCC 18-0427 and GZCC 20-0426.
Notes: Xylolentia aseptata resembles X. reniformis in the morphology of conidiogenous cells and conidia with comparable conidial dimension [3–5.5 × 1.8–3 µm (\({\overline{\text{x}}}\) = 4 × 2.3 µm) vs. 3.2–4.6 × 1.8–3 µm], but conidiophores of the former are longer (95–215 µm vs. 65–160 µm) (Yuan et al. 2020). They were collected in Guizhou Province, China. Xylolentia aseptata grew on submerged decaying wood in a freshwater stream, while X. reniformis occurred on a dead twig from the waterside. Comparisons of their LSU, ITS, and TEF1α sequences showed 99.4% (five bp differences), 97.9% (12 bp differences) and 98% (18 bp with two amino acids differences) sequence similarity, respectively. The RPB2 sequence is unavailable for X. reniformis. The hyaline, ellipsoidal to reniform conidia of X. aseptata distinguish it from X. brunneola which has hyaline to pale brown, ellipsoidal, subglobose to obovoid conidia.
In the phylogenetic analysis (Fig. 53), a non-type strain Linkosia multiseptum (HKUCC 10825) was sister to Xylolentia aseptata (GZCC 20-0424 and GZCC 20-0426). Linkosia multiseptum has brown, obclavate, rostrate, distoseptate, thick-walled conidia and reduced conidiophores. It differs from the phaeoisaria-, idriella-, veronaea-like and Xylolentia asexual morphs in Rhamphoriaceae. Réblová and Štěpánek (2018) questioned the placement of L. multiseptum in Rhamphoriaceae, and Linkosia was shown to be polyphyletic (Shenoy et al. 2006). Without an illustration or description of the strain HKUCC 10825, it is surmised that it was a contaminant when isolating Xylolentia conidia. We retain Linkosia multiseptum in the family until its identification is resolved.
Savoryellales Boonyuen, Suetrong, Sivichai, K.L. Pang & E.B.G. Jones
Notes: Savoryellales was established as monotypic and positioned in Hypocreomycetidae based on a combined dataset of six ribosomal and protein-coding loci (Boonyuen et al. 2011). With further evidence from phylogenetic and molecular clock analyses, Savoryellales was referred to Savoryellomycetidae (Hongsanan et al. 2017). Savoryellaceae was introduced by Ranghoo (1998) lacking a description. It was formally published by Jaklitsch and Réblová (2015). Species in Savoryellales are saprobic on decaying wood from terrestrial and aquatic habitats, including marine, brackish, and freshwater environments (Minoura and Muroi 1978; Koch 1982; Jones and Hyde 1992; Chang et al. 1998; Hyde and Jones 1998; Abdel-Wahab and Jones 2000; Jones et al. 2016; Dayarathne et al. 2019). The type species Savoryella lignicola was described from Scots pine test blocks in a water-cooling tower in the UK (Jones and Eaton 1969).
Savoryellaceae Jaklitsch & Réblová
Notes: Savoryellaceae comprises four holomorphic genera, Ascotaiwania, Canalisporium, Neoascotaiwania and Savoryella, and two asexual genera, Bactrodesmium and Dematiosporium (Réblová et al. 2020a). The sexual-asexual connections were confirmed by cultivation and/or molecular data (Chang et al. 1998; Ranghoo and Hyde 1998; Sivichai et al. 1998; Sri-indrasutdhi et al. 2010; Boonyuen et al. 2011; Réblová et al. 2016c; 2020a; Yang et al. 2016b; Hernández-Restrepo et al. 2017; Zhang et al. 2019).
Savoryellaceae taxa share immersed to superficial, dark perithecial ascomata with elongated, dark or subhyaline neck, often oblique or lying horizontally on the host with the neck facing upwards, partly deliquescing paraphyses, non-amyloid apical annulus of unitunicate asci and ellipsoidal to fusiform, transversely septate, versicolor ascospores with hyaline end cells and brown median cells. The type genus Savoryella resembles Ascotaiwania in the morphology of sexual morphs, which makes their identification challenging. A survey of predominant diagnostic characters on ascospore septation, apex of asci and paraphyses, and researchers’ interpretation were summarized in Réblová et al. (2016c). Asexual morphs in the family were characterized by sporodochial conidiomata or effuse colonies, mononematous, reduced, or short conidiophores, monoblastic conidiogenous cells and subglobose, ellipsoidal, obovoid or pyriform, pigmented conidia with transverse or muriform septa.
Aquabispora J. Yang, E.B.G. Jones & K.D. Hyde, gen. nov.
Index Fungorum number: IF559828; Facesoffungi number: FoF12839
Etymology: referring to the aquatic habitats and 2-spored asci of the type species.
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata perithecial, superficial, subglobose to obpyriform, dark brown to black, setose, papillate. Ostiole periphysate. Setae lanceolate, dark brown, scattered over ascoma densely clustered around the ostiole, originating from surface cells of ascomata wall. Ascomatal wall coriaceous, 2-layered. Paraphyses abundant, persistent, septate. Asci unitunicate, broadly cylindrical to clavate, stipitate, straight or slightly curved, rounded or papillate at the apex, 2-spored, with a non-amyloid apical apparatus which has a central pore and retraction of plasmalemma. Ascospores uniseriate, cylindrical-ellipsoidal, hyaline when young, brown when mature, muriform, often surrounded by a mucilaginous sheath.
Type species: Aquabispora setosa J. Yang, E.B.G. Jones & K.D. Hyde
Notes: Aquabispora is similar to Boerlagiomyces in having superficial, setose ascomata and muriform ascospores with a mucilaginous sheath (Crane et al. 1998). However, Aquabispora has 2-spored asci while Boerlagiomyces produces 8-spored asci except for B. lacunosisporus with 2–4-spored asci. Boerlagiomyces velutinus, the generic type, differs from Aquabispora by ascomata growing under a subiculum, clavate, 8-spored asci lacking an apical apparatus, and cylindrical–fusoid to clavate ascospores. Among Boerlagiomyces species, molecular data is only available for a reference specimen of B. macrospora, which was attributed to Tubeufiaceae (Tubeufiales, Dothideomycetes) (Doilom et al. 2017). Based on an LSU phylogeny, a non-type strain Boerlagiomyces websteri (BCC 3834) clustered in Rhytismataceae (Leotiomycetes) which was unreliable and lacked a morphological description (Kodsueb et al. 2006a; Boonmee et al. 2014). In our phylogenetic analysis (Fig. 53), Aquabispora setosa nested within Savoryellaceae (Savoryellales, Sordariomycetes) as a sister taxon to Aquabispora sp. (MFLU 18-1002), which was initially identified as Boerlagiomyces websteri (unpublished). In this study, we introduce a new genus Aquabispora to accommodate Boerlagiomyces websteri, B. grandisporus and a new species based on the morphology and molecular DNA data.
Aquabispora setosa J. Yang, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF559829; Facesoffungi number: FoF12840; Fig. 92
Aquabispora setosa (HKAS 124629, holotype) a, b Ascomata on wood. c Section of an ascoma. d, e Section of peridium. f Surface view of peridium. g Seta. h, i Paraphyses. j–o Asci. p–s Ascospores. t Ascospore in Indian ink. u Apical part of an ascus. v Basal part of an ascus. w Germinated ascospore. x, y Culture, x from above, y from below. Scale bars: c, j, l–o = 100 μm, g, k, p–u, w = 50 μm, e, f, h, i, v = 30 μm, d = 20 μm
Etymology: referring to the setose ascomata.
Holotype: HKAS 124629
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Undetermined. Sexual morph: Ascomata 280–450 μm high, 250–350 μm diam., scattered or aggregated, superficial, perithecial, subglobose to obpyriform, dark brown, ostiolate, setose, papillate. Ostiole periphysate. Setae lanceolate, stiff, dark brown, 1–2-septate, scattered over ascoma densely clustered around the ostiole, 150–230 µm long, 6–8 µm wide at base, originating from surface cells of ascomata wall. Ascomatal wall coriaceous, 25–55 µm thick, consisting of multi-layered cells of textura angularis, with pale brown, thick-walled cells to hyaline, thin-walled, elongated cells from outer to inner layer. Paraphyses abundant, persistent, septate, hyaline, some constricted at the septa, thin-walled, 3.5–8 µm wide. Asci (215–)245–345(–370) × 40–90 µm (\({\overline{\text{x}}}\) = 290 × 65 µm, n = 20), few, broadly cylindrical-clavate, straight or slightly curved, rounded or papillate at the apex, 2-spored, rarely 1-spored, with a non-amyloid apical apparatus which has a central pore and retraction of plasmalemma, short stalked. Ascospores (88–)100–140(–148) × (38–)45–62 µm (\({\overline{\text{x}}}\) = 118 × 52 µm, n = 40), uniseriate, cylindrical, cylindrical-ellipsoidal, broadly rounded at both ends, hyaline when young, brown when mature, muriform, dictyosporous with 7–11 (mostly 9) transverse septa and 1–3 longitudinal septa, smooth-walled, thin-walled, surrounded by a mucilaginous sheath with undulate edge.
Culture characteristics: Ascospores germinating on WA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium slow-growing, reaching 5–10 mm diam. after 1 month at 25 °C in dark, circular, with dense, mostly immersed, dark green mycelium on the surface; in reverse dark green to black with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS9-1 (HKAS 124629, holotype), ex-type culture GZCC 20-0492.
Notes: Aquabispora setosa resembles A. websteri and A. grandispora in having superficial, setose ascomata with a short neck, cylindrical-clavate, 2-spored asci with a cap-like apical apparatus, and cylindrical or ellipsoidal, muriform ascospores, hyaline at first becoming brown and with a mucilaginous sheath (Shearer and Crane 1995; Stanley and Hyde 1997). Aquabispora setosa has the same morphology as A. websteri except for producing larger asci [(215–)245–345(–370) × 40–90 µm (\({\overline{\text{x}}}\) = 290 × 65 µm) vs. 164–214(–278) × 20–43 µm] and ascospores [(88–)100–140(–148) × (38–)45–62 µm (x̅ = 118 × 52 µm) vs. 66–92(–107) × 26–35 µm] (Shearer and Crane 1995). Aquabispora grandispora can be distinguished by hyaline and cream-colored ascomata, ellipsoidal ascospores with (13–)14(–16) transverse septa, 5–7 longitudinal and oblique longitudinal septa, and darker poles, while A. setosa and A. websteri have brown ascomata, cylindrical ascospores with broadly rounded and obtuse ends, and mostly nine transverse septa and 1–3 longitudinal septa (Shearer and Crane 1995).
In Savoryellaceae, Aquabispora setosa resembles Ascotaiwania latericolla, Ascotaiwania lignicola and Dematiosporium aquaticum in having muriform spores (Chang 2001; Luo et al. 2019; Réblová et al. 2020a). However, they are phylogenetically distinct taxa (Fig. 53). Savoryella paucispora is similar to A. setosa in having 2-spored asci, but differs by immersed ascomata, poorly developed paraphyses and fusiform, 3-septate ascospores, constricted at the septa with dark middle cells and hyaline end cells (Cribb and Cribb 1960).
Aquabispora grandispora (S.J. Stanley & K.D. Hyde) J. Yang, E.B.G. Jones & K.D. Hyde, comb. nov.
Index Fungorum number: IF559830; Facesoffungi number: FoF12841
Basionym: Boerlagiomyces grandisporus S.J. Stanley & K.D. Hyde, Mycol Res 101(5): 635 (1997)
Holotype: Philippines, Negros Occidental, near Bacolod, Bario Alegria, on wood submerged in a river, 1 Jan 1994, K.D. Hyde & E. Arimas (HKU(M) 2978).
Notes: This species was saprobic on leaves or wood from terrestrial and freshwater habitats. It has been reported in Australia (Hyde and Goh 1998), China (Goh and Hyde 1999), India (Sridhar et al. 2011), Mexico (García-García et al. 2013), Philippines (Stanley and Hyde 1997; Cai et al. 2003a) and Thailand (Kodsueb et al. 2008).
Aquabispora websteri (Shearer & J.L. Crane) J. Yang, E.B.G. Jones & K.D. Hyde, comb. nov.
Index Fungorum number: IF559831; Facesoffungi number: FoF12842
Basionym: Boerlagiomyces websteri Shearer & J.L. Crane, Mycologia 87(6): 876 (1995)
Holotype: USA, Illinois, Johnson Co., Cache River at foot bridge to Heron Pond, UTM Zone 16, 330390mE, 4135430mN, a dried culture isolated from muddy, submerged, decorticated wood, 3 Oct 1992, C.A. Shearer and J.L. Crane, A-59-1 (ILLS 51611; isotypes, NY, DAOM; culture, ATCC 96580).
Notes: The fungus has been recorded on submerged wood in the USA, cherry stones in Hungary, and fruit and seeds in Thailand (Shearer and Crane 1995; Tóth 2009). It can produce several extracellular degradative enzymes on different solid media (Abdel-Raheem and Shearer 2002).
Sordariales Chadef. ex D. Hawksw. & O.E. Erikss.
Notes: Sordariales was introduced by Hawksworth and Eriksson (1986). Huhndorf et al. (2004) redefined the order based on the molecular analysis using LSU sequence. Currently, the order comprises Bombardiaceae, Chaetomiaceae, Diplogelasinosporaceae, Lasiosphaeriaceae, Lasiosphaeridaceae, Naviculisporaceae, Neoschizotheciaceae, Podosporaceae, Strattoniaceae, Sordariaceae and Zygospermellaceae (Huang et al. 2021).
Neoschizotheciaceae S.K. Huang & K.D. Hyde
Notes: Neoschizotheciaceae was established by Huang et al. (2021) to accommodate taxa in Schizotheciaceae clade. Neoschizotheciaceae is characterized by immersed to semi-immersed or superficial, obpyriform or ovoid ostiolate ascomata, cylindrical to clavate asci and ellipsoidal ascospores, sometimes with long or short cylindrical or lash-like gelatinous appendages (Huang et al. 2021).
Cercophora Fuckel
Notes: Cercophora was introduced by Fuckel (1870) with C. mirabilis as the type. The systematic placement of Cercophora species is polyphyletic in different families within Sordariales (Miller and Huhndorf 2004; Kruys et al. 2015; Hyde et al. 2020a; Huang et al. 2021). The genus is characterized by membranaceous to carbonaceous, ostiolate, papillate ascomata, asci with an apical ring and hyaline, cylindrical ascospores with a brown, swollen head at maturity (Miller and Huhndorf 2004; Catania et al. 2011).
Cercophora caudata (Sacc.) N. Lundq., Symb bot upsal 20(1): 92 (1972)
Index Fungorum number: IF310543; Facesoffungi number: FoF05476; Fig. 93
Basionym: Sphaeria caudata Curr., Trans Linn Soc Lond 22: 320 (1859)
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: see Luo et al. (2019). Sexual morph: Ascomata 350–500 μm diam., scattered or aggregated, immersed to semi-immersed, perithecial, subglobose to ovoid, dark brown to black, ostiolate, glabrous, with a cylindrical to conical neck. Ostiole periphysate. Ascomatal wall coriaceous, of textura angularis in surface view. Paraphyses abundant, persistent, septate, hyaline, usually constricted at the septa, thin-walled, 3–11 µm wide. Asci 187–329 × 11–35 µm (\({\overline{\text{x}}}\) = 245 × 20 µm, n = 30), cylindrical to clavate, with an obtuse apex, 8-spored, hyaline, with a non-amyloid apical annulus and usually with a subapical globule. Ascospores overlapping 2–3-seriate, usually flexuous, smooth-walled, guttulate, septate, usually with a filiform appendage at both ends, hyaline and cylindrical when young of 59–85 × 4.5–5.5 µm (\({\overline{\text{x}}}\) = 70 × 5 µm, n = 20), apical cells becoming swollen and darker during mature; when mature, swollen head ovoid, truncate at the base and tapering at the apex, dark olivaceous brown to dark brown, aseptate, 16–22 × 9–12 µm (\({\overline{\text{x}}}\) = 19 × 10.5 µm, n = 20); pedicel hyaline to pale brown, cylindrical, curved, uniseptate, 43–50 × 3.7–5.5 µm (\({\overline{\text{x}}}\) = 47 × 4.8 µm, n = 20); appendages (19.5–23 µm long) and pedicel generally collapse and disappear with age.
Culture characteristics: Ascospores germinating on WA medium within 24 h. Germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm diam. after 3 weeks at 25 °C in dark, circular, with dense, velvety, olivaceous mycelium on the surface; in reverse dark olivaceous with entire margin.
Material examined: CHINA, Guizhou Province, Anshun City, Gaodang Village, 26.071° N, 105.698° E, on decaying wood submerged in Suoluo River, 19 October 2016, J. Yang, GD24-6 (HKAS 112635); living cultures MFLUCC 17-0236 and GZCC 17-0053.
Notes: Cercophora caudata was introduced by Lundqvist (1972) as a sexual morph. It was commonly found on rotten wood and occasionally from dung (Lundqvist 1972; Mustafa and Abdullah 2011). Miller and Huhndorf (2004) introduced Immersiella to accommodate Cercophora caudata and Lasiosphaeria immersa which clustered together in the phylogenetic analyses. Cai et al. (2005) found that C. caudata is phylogenetically close to Podospora. Luo et al. (2019) reported the asexual morph for this species, and their phylogenetic analysis showed that C. caudata clustered with other Cercophora species in Lasiosphaeriaceae. In the current delimitation, C. caudata is treated as a member of Cercophora within Neoschizotheciaceae (Huang et al. 2021).
The morphology of our collection fits well with the diagnosis of C. caudata (Lundqvist 1972; Mustafa and Abdullah 2011). In the phylogenetic analysis (Fig. 53), our collection (GZCC 17-0053) grouped with three strains of C. caudata (MFLUCC 16-1271, MFLUCC 17-0475 and CBS 606.72). Hence, based on the phylogeny and morphology, we identify our collection as C. caudata which was recollected from freshwater habitats in China.
Sporidesmiales Crous
Notes: Divergence time estimates suggested that Sporidesmiaceae can be upgraded to an ordinal level as Sporidesmiales (Hongsanan et al. 2017; Hyde et al. 2017a). Crous et al. (2018b) established the monotypic order Sporidesmiales based on LSU sequence data. The order is classified in Diaporthomycetidae, Sordariomycetes (Hyde et al. 2020a, 2021a).
Sporidesmiaceae Fr.
Notes: Sporidesmiaceae was established by Fries (1849) but rarely used. Su et al. (2016a) resurrected the name and designated a monophyletic clade of Sporidesmium species as Sporidesmiaceae sensu stricto.
Sporidesmium Link
Notes: Sporidesmium was introduced by Link (1809) with S. atrum as the type species. The genus is heterogeneous with nearly 500 epithets listed in Index Fungorum (2022). Shenoy et al. (2006) found that Sporidesmium sensu lato was polyphyletic across the Ascomycota. The genus was restricted to a monophyletic clade as Sporidesmium sensu stricto by Su et al. (2016a). It is characterized by macronematous, mononematous, erect or somewhat repent, cylindrical, brown, septate conidiophores, monoblastic, holoblastic, percurrent conidiogenous cells and ovoid, obpyriform or rostrate, euseptate or distoseptate conidia with or without an appendage or mucilaginous sheath (Su et al. 2016a; Zhang et al. 2017b; Yang et al. 2018a; Hyde et al. 2020a).
Sporidesmium tratense J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF559832; Facesoffungi number: FoF12844; Fig. 94
Sporidesmium tratense (MFLU 22-0070, holotype) a Colony on wood. b–d Conidiophores and conidia. e–g Conidiophores with conidiogenous cells. h Conidium on a conidiogenous cell bearing a cyathiform cup. i–l Conidia. m Germinated conidium. n, o Culture, n from above, o from below. Scale bars: a = 200 µm, b–h = 30 µm, i–m = 20 µm
Etymology: referring to the collecting site of the species at Trat Province in Thailand.
Holotype: MFLU 22-0070
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, dark brown, scattered or in small groups, glistening. Mycelium mostly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, solitary or caespitose, straight or slightly flexuous, cylindrical, septate, smooth-walled, unbranched, mid to dark brown, slightly paler at the apex, (53–)85–120(–160) × 5–8.5 µm (\({\overline{\text{x}}}\) = 100 × 6 µm, n = 20). Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical or lageniform, brown, 11.5–20 × 4.5–7 µm, truncate at the apex, with up to three lageniform percurrent proliferations, darkened at the apex and percurrent loci, sometimes bearing a brown cyathiform cup at the percurrently proliferating loci. Conidia acrogenous, solitary, obpyriform or obclavate, rostrate, 4–7-euseptate, smooth-walled, mid to dark brown, with a pale brown to subhyaline apical cell, 29–43 × 11–15 µm (\({\overline{\text{x}}}\) = 36 × 12.8 µm, n = 20), guttulate, truncate at the base with a darkened scar, thickened and darkened at the septa. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h. Germ tubes produced from the apex. Colonies on MEA medium reaching 10–15 mm diam. after 2 weeks at 25 °C in natural light, circular, wrinkled, with dense white mycelium on the surface, sparser to the edge; in reverse with a yellow middle and paler entire margin.
Material examined: THAILAND, Trat Province, Amphoe Ko Chang, 12.133° N, 102.633° E, on decaying wood submerged in a freshwater stream, 27 April 2017, Y.Z. Lu, YJT20-2 (MFLU 22-0070, holotype; HKAS 112156, isotype), ex-type cultures MFLUCC 17-2392 and GZCC 20-0393.
Notes: In the phylogenetic analysis (Fig. 53), Sporidesmium tratense is basal to S. dulongense, S. lageniforme, S. submersum and S. thailandense with strong statistical support (100% MLBS/1.0 PP). Sporidesmium tratense is distinguished from other species in the genus by the swollen, globose to cup-like cells at the percurrently proliferating loci of conidiogenous cells. Sporidesmium tratense resembles S. dulongense, S. lageniforme and S. submersum in having obpyriform or lageniform, dark brown conidia with a taper and hyaline apex (Su et al. 2016a; Luo et al. 2019; Hyde et al. 2020a). However, the conidia of S. tratense are shorter than that of S. dulongense (29–43 μm vs. 50–58 μm) and S. submersum (29–43 μm vs. 42–52 µm). Besides, S. dulongense differs from S. tratense by longer and narrower conidial apical cells, and S. submersum differs by shorter conidiophores [59–72 μm vs. (53–)85–120(–160)] (Su et al. 2016a; Hyde et al. 2020a). In addition, S. lageniforme has an overlapping dimension of conidiophores (105–141 μm) and larger conidia (38–48 × 13–17 μm vs. 29–43 × 11–15 µm) compared with S. tratense (Luo et al. 2019). The ITS sequence of S. tratense differs from S. dulongense and S. lageniforme by 36 bp (total 518 bp, 11 gaps) and 41 bp (total 549 bp, ten gaps), respectively. The ITS sequence of S. submersum is unavailable.
Sporidesmium versicolor J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF900056; Facesoffungi number: FoF12843; Fig. 95
Etymology: referring to the versicolor conidia which are subhyaline in the apical cell and brown in lower cells.
Holotype: MFLU 15-1139
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, dark brown, scattered or in small groups, glistening. Mycelium partly immersed, partly superficial, composed of septate, smooth-walled, pale brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, solitary or caespitose, straight or slightly flexuous, cylindrical, smooth-walled, septate, unbranched, dark brown, slightly narrower and paler towards the apex, 50–130 × 3.5–5 µm (\({\overline{\text{x}}}\) = 95 × 4.2 µm, n = 15). Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical or lageniform, brown, 12–22 × 4.5–6 µm, truncate at the apex with a darkened scar, sometimes elongating percurrently. Conidia acrogenous, ovoid to obpyriform, with a conical apical cell, mostly 3-septate, rarely 4-septate, smooth-walled, brown, with apical cell pale brown to subhyaline, 19–25 × 8.5–11.5 µm (\({\overline{\text{x}}}\) = 22 × 10 µm, n = 20), guttulate, truncate at the base, thickened and darkened at the septa, rarely with a mucilaginous sheath at the apex. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on PDA medium within 24 h and germ tubes produced from one or both ends. Colonies on MEA medium reaching 10–15 mm in 1 month at 25 °C in natural light, circular, with velvety, white mycelium on the surface; in reverse dark yellow with undulate margin.
Material examined: THAILAND, Chiang Rai, Tham Luang Nang Non Cave, on decaying wood submerged in a freshwater stream, 25 November 2014, J. Yang, site3-4-4 (MFLU 15-1139, holotype), ex-type cultures MFLUCC 15-0604 and GZCC 16-0009.
Notes: Sporidesmium versicolor resembles S. cookei in having solitary or caespitose, brown conidiophores, monoblastic conidiogenous cells elongating percurrently and 3-septate conidia in similar dimension, with a paler apical cell and brown lower cells (Ellis 1958). However, conidiophores of S. versicolor are shorter than that of S. cookei (50–130 µm vs. 70–200 µm) (Ellis 1958). Sporidesmium versicolor has obpyriform, smooth-walled conidia, while S. cookei produces obpyriform or obclavate, smooth-walled or occasionally verruculose conidia (Ellis 1958). The mucilaginous sheath of S. versicolor was not observed in S. cookei. Molecular data is not available for S. cookei. In the phylogenetic analysis (Fig. 53), S. versicolor (MFLUCC 15-0604) clustered with the strain HKUCC 10836 as a sister taxon. HKUCC 10836 was initially identified as S. parvum without an associated description or illustration (Shenoy et al. 2006). HKUCC 10836 is only available with an LSU sequence with 99.88% (840/841 bp) similarity to S. versicolor. In this study, we recognize the strain HKUCC 10836 as an unknown species in Sporidesmium, and further molecular evidence is needed to confirm its relationship with S. versicolor.
Xenospadicoidales Hern.-Restr., J. Mena & Gené
Notes: The monotypic order Xenospadicoidales was introduced by Hernández-Restrepo et al. (2017) with Xenospadicoidaceae as the type family, based on an LSU sequence analysis. Later, the multigene-based phylogeny and divergence time analysis further confirmed Xenospadicoidales as a strongly supported monophyletic group within Diaporthomycetidae, Sordariomycetes (Réblová et al. 2018; Luo et al. 2019; Hyde et al. 2020a).
Xenospadicoidaceae Hern.-Restr., J. Mena & Gené
Notes: Hernández-Restrepo et al. (2017) established Xenospadicoidaceae to accommodate two genera Xenospadicoides and Pseudodiplococcium. Réblová et al. (2018) redefined the family with Xenospadicoides and Pseudodiplococcium synonymized to Spadicoides and included Calyptosphaeria, Lentomitella, and Torrentispora in the family. Lentomitellaceae, typified by Lentomitella, was therefore synonymized to Xenospadicoidaceae (Réblová et al. 2018). Luo et al. (2019) introduced Neospadicoides, and Lad et al. (2022) added Gangliostilbe to the family. Thus, six genera, Calyptosphaeria, Gangliostilbe, Lentomitella, Neospadicoides, Spadicoides, and Torrentispora, are accepted in Xenospadicoidaceae.
Neospadicoides Z.L. Luo, K.D. Hyde & H.Y. Su
Notes: Neospadicoides was introduced by Luo et al. (2019) with N. lignicola as the type species. Neospadicoides comprises six species with five reported from freshwater habitats (Luo et al. 2019; Bao et al. 2021b; Hyde et al. 2021b).
Neospadicoides biseptata J. Yang, L.L. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559855, Facesoffungi number: FoF12845; Fig. 96
Etymology: referring to the 2-septate conidia.
Holotype: GZAAS 20-0343
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, brown to dark brown, scattered, glistening. Mycelium partly superficial, partly immersed, composed of septate, branched, smooth-walled, pale brown hyphae. Conidiophores macronematous, mononematous, erect, solitary or in small groups, unbranched, straight, or slightly flexuous, cylindrical, 7–10-septate, smooth-walled, dark brown, slightly paler and tapering towards the apex, 125–160(–240) × 4–7 μm (\({\overline{\text{x}}}\) = 142.5 × 5.6 μm, n = 15). Conidiogenous cells monoblastic, integrated or discrete, terminal, and intercalary, determinate, ovoid, brown, 5–9.5(–12) × 2.5–4.5 μm (\({\overline{\text{x}}}\) = 7.5 × 3.3 μm, n = 25), darkened at the rounded base, arranged sympodially on conidiophores, elongating percurrently. Conidia acrogenous, solitary, obovoid, 2-septate, smooth-walled, subhyaline to pale brown or pale grayish green, 8–13 × 3–5 μm (\({\overline{\text{x}}}\) = 10 × 4.2 μm, n = 30), guttulate, with a small truncate base. Sexual morph: Undetermined.
Cultural characteristics: Conidia germinated on WA medium within 12 h. Colonies on PDA medium slow growing, reaching 10–15 mm diam. in 1 month at 25 °C in dark, circular, with velvety, grayish brown mycelium on the surface; in reverse dark brown with filiform margin.
Material examined: CHINA, Guizhou Province, Dushan District, Duliujiang Wetland, 25.917° N, 107.617° E, on decaying wood submerged in a freshwater stream, 7 July 2018, L.L. Liu, 18D-18 (GZAAS 20-0343, holotype), ex-type culture GZCC 19-0448; ibid, 18D-12 (GZAAS 20-0432, paratype), ex-paratype culture GZCC 19-0537; ibid, 26 Aug 2017, J. Yang, SG10-1 (HKAS 124635, paratype), ex-paratype cultures MFLUCC 18-0421 and GZCC 20-0419.
Notes: Among Neospadicoides species, N. biseptata, N. aquatica and N. yunnanensis possess mostly 2-septate conidia while others have 3-septate conidia. Neospadicoides aquatica is distinguished from N. biseptata by integrated cylindrical conidiogenous cells and mid-brown, fusiform to ellipsoidal conidia with darkened septa (Luo et al. 2019). Neospadicoides biseptata and N. yunnanensis are similar in morphology, having discrete ovoid conidiogenous cells and pale brown obovoid conidia. Conidiophores of both species are in a similar dimension. However, N. biseptata has slightly longer but narrower conidia (8–13 × 3–5 μm vs. 7.5–10.5 × 4–6 μm) and longer conidiophores (125–160(–240) μm vs. 113–153 μm) than that in N. aquatica (Luo et al. 2019). In the phylogenetic analysis (Fig. 53), N. biseptata (GZCC 19-0448, GZCC 19-0537 and GZCC 20-0419) formed a sister clade to N. yunnanensis (DLUCC 1499) with strong statistical support (100 MLBS/1.0 PP). The LSU, ITS, and RPB2 sequences of N. biseptata (GZCC 19-0448, ex-type) and N. yunnanensis (DLUCC 1499, ex-type) showed 98.16% (745/759 bp, two gaps), 96.91% (502/518 bp, one gap) and 93.09% (997/1071 bp, four gaps) similarity, respectively.
Xylariales genera incertae sedis
Stanjehughesia Subram.
Notes: Given the diagnostic value of euseptate and distoseptate conidia, absence, or presence of conidiophores and percurrent proliferation of conidiophores, Subramanian (1992) redefined the heterogenous genus Sporidesmium with the majority of species distributed in seven genera. Stanjehughesia is one of the genera introduced in Subramanian’s reassessment of Sporidesmium. Stanjehughesia species lack conidiophores and have obclavate to obclavate-rostrate, euseptate, smooth or verrucose conidia that are produced directly on simple, monoblastic conidiophores (Subramanian 1992; Wu and Zhuang 2005; Seifert et al. 2011). So far, Stanjehughesia comprises 20 species (Hsieh et al. 2021). The sexual morphs Umbrinosphaeria caesariata (synonym: Chaetosphaeria caesariata) and Miyoshiella larvata were linked to Stanjehughesia based on cultural studies (Réblová 1999). Linkosia is similar to Stanjehughesia in the reduced conidiophores and conidial shape and color but can be distinguished by distoseptate conidia (Hernández-Gutiérrez and Sutton 1997). Phylogenetic studies revealed that both genera are polyphyletic (Shenoy et al. 2006; Su et al. 2016a; Yang et al. 2018a; Hyde et al. 2020a; Hsieh et al. 2021). Thus, the generic delimitation and treatment of Stanjehughesia, Linkosia and other questionable sporidesmium-like taxa await resolution.
Stanjehughesia aquatica J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559856; Facesoffungi number: FoF12846; Fig. 97
Stanjehughesia aquatica (HKAS 112612, holotype) a, b Colony on wood. c–f Conidiogenous cells with conidia. g, h Conidiogenous cells. i–l Conidia. m Conidiogenous cell with a conidium. n Germinated conidium. o, p Culture, o from above, p from below. Scale bars: c–f, i–l, n = 30 μm, m = 20 μm, g, h = 10 μm
Etymology: referring to the aquatic habitat of the species.
Holotype: HKAS 112612
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, dark brown, scattered or in small groups, glistening. Mycelium mostly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores reduced to conidiogenous cells. Conidiogenous cells monoblastic, integrated, terminal, determinate, erect, solitary or caespitose, straight, cylindrical or lageniform, aseptate, smooth-walled, dark brown, truncated at the apex, 7–12 × 2–3.5 µm (\({\overline{\text{x}}}\) = 9.2 × 2.7 µm, n = 20). Conidia acrogenous, solitary, rostrate, 11–15-euseptate, verrucose, yellowish brown, pale brown to subhyaline at the apex, 74–135 × 8–12.5 µm (\({\overline{\text{x}}}\) = 106 × 10 µm, n = 30), guttulate, rounded at the apex and truncated at the base. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h and germ tubes produced from both ends. Colonies on PDA medium reaching 10–15 mm in 2 weeks at 25 °C in dark, circular, raised, waxy-mucoid, producing gelatinous drops on the surface, with dense, finely floccose, grayish brown mycelium on the surface; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS29-2 (HKAS 112612, holotype; HKAS 125934, isotype), ex-type culture GZCC 20-0506.
Notes: In the phylogenetic tree (Fig. 53), Stanjehughesia aquatica (GZCC 20-0506) clustered with S. polypora (NN47796) within Xylariales clade. They are distantly related to Stanjehughesia species in Chaetosphaeriaceae. Comparisons of the LSU and ITS sequences of S. aquatica and S. polypora showed 98.42% (810/823 bp) and 88.73% (488/550 bp, 29 gaps) similarity, respectively. Stanjehughesia polypora is distinguished from S. aquatica by conspicuous germination pores in almost every conidial cell (Wu and Zhuang 2005). The rostrate conidia with strongly narrower upper part of S. aquatica resemble those of S. decorosa, S. fusiformis, S. jiangxiensis and S. kaohsiungensis. Stanjehughesia aquatica and S. decorosa have verrucose conidia, but the latter is distinct in having a mucilaginous sheath at the conidial apex and sometimes percurrently elongating conidiogenous cells (McKenzie 1995). Stanjehughesia fusiformis can be distinguished from S. aquatica by pale brown to brown, smaller conidia (65–85 × 7–8 µm vs. 74–135 × 8–12.5 µm) with constricted septa (Wu and Zhuang 2005). Stanjehughesia jiangxiensis is similar to S. aquatica in conidial shape and size but different by smooth-walled conidia and larger conidiogenous cells (9.5–22 × 3.5–4 µm vs. 7–12 × 2–3.5 µm) (Ma 2016). Stanjehughesia kaohsiungensis was introduced with LSU and ITS sequences which belongs to Chaetosphaeriaceae (Hsieh et al. 2021).
Sordariomycetes families incertae sedis
Acrodictyaceae J.W. Xia & X.G. Zhang
Notes: Acrodictyaceae is a monotypic family comprising saprobic dematiaceous hyphomycetes from terrestrial and freshwater environments (Ellis 1961; Cai et al. 2002c; Xia et al. 2017; Luo et al. 2019). The family is characterized by macronematous, mononematous conidiophores, monoblastic conidiogenous cells often elongating percurrently and brown, obovoid to pyriform, muriform conidia with schizolytic conidial secession. Previous phylogenetic studies mainly inferred from LSU and ITS sequences revealed an unstable systematic placement of Acrodictyaceae within Diaporthomycetidae (Xia et al. 2017; Luo et al. 2019; Hyde et al. 2020a, 2021a; Dong et al. 2021a).
Acrodictys M.B. Ellis
Notes: Acrodictys is a heterogeneous assemblage established with a broad generic concept (Ellis 1961). The genus is delimited as having single or clustered conidiophores with proliferation and variable-shaped, brown conidia with transverse and longitudinal septa and blown-out ends. More than 30 epithets were referred to Acrodictys. Segregations of Acrodictys were established based on branched or unbranched, mono- or synnematous conidiophores, morphology of conidiogenous cells and conidial shape, color, and septa details (Baker et al. 2001, 2002a, b; Baker and Morgan-Jones 2003; Gams et al. 2009; Zhao et al. 2011). Many Acrodictys species were transferred to allied genera, e.g., Junewangia, Pseudoacrodictys, Rhexoacrodictys, Septosporiopsis and Synnemacrodictys. Acrodictys sensu stricto currently contains more than 20 species, with some remain phylogenetically problematic (Baker et al. 2002a, b; Baker and Morgan-Jones 2003; Xia et al. 2017; Chethana et al. 2021; Index Fungorum 2022). Acrodictys species were collected from fungal colonies or decaying leaves, culms, and branches from terrestrial and freshwater habitats and distributed worldwide. Xia et al. (2017) assessed the systematic placement of Acrodictys and provided the molecular DNA data of several new and old acrodictys-like species. It showed that Acrodictys clustered within Diaporthomycetidae and separated from similar genera Junewangia and Rhexoacrodictys (Xia et al. 2017). However, sequences from the holotype of the old Acrodictys species and sexual morph connections are still unavailable. Thus, epitypifications and new materials are needed to understand the phylogenetic placement of Acrodictys.
Acrodictys chishuiensis J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559869; Facesoffungi number: FoF12849; Fig. 98
Etymology: referring to the collecting site at Chishui City.
Holotype: HKAS 112618
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies on wood effuse, hairy, dark brown, scattered, glistening. Mycelium mostly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly flexuous, cylindrical, septate, smooth-walled, unbranched, dark brown, slightly paler, and narrower towards the apex, 65–135 × 4–5.4 µm. Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical, lageniform or doliiform, brown, truncate at the apex, sometimes elongating percurrently. Conidia acrogenous, solitary, broadly pyriform, muriform, usually with 4–5 transverse septa and 2–3 longitudinal septa, smooth-walled, brown to dark brown, 23–29 × 14–21 µm (\({\overline{\text{x}}}\) = 25.8 × 17.5 µm, n = 20), constricted at the septa, with conspicuous pores at the septa, truncate at the base, basal cell obconical often disintegrating after release, the second basal cell cuneiform. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h, germ tubes produced from the base. Colonies on PDA medium reaching 10–15 mm in 1 month at 25 °C in dark, with dense mycelium on the surface, brown in the middle, paler towards the edge; in reverse dark brown with white entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS42-2-1 (HKAS 112618, holotype), ex-type culture GZCC 20-0513.
Notes: In the phylogenetic analysis (Fig. 53), Acrodictys chishuiensis (GZCC 20-0513) formed a distinct branch within Acrodictys with weak support. Acrodictys chishuiensis is similar to A. elliptica and A. peruamazonensis in having lageniform or cylindrical conidiogenous cells and ellipsoidal to obovoid conidia with mostly 3-column cells and paler basal cells (Matsushima 1993; Manoharachary et al. 2006). Conidia of A. elliptica are 2–3 transversely septate, brownish black in the central part and pale brown at the distal end. Acrodictys peruamazonensis has light to mid-brown conidia with 4–6 transverse septa. Acrodictys chishuiensis differs from A. elliptica and A. peruamazonensis by yellowish brown to dark brown conidia with 4–5 transverse septa and the presence of septal pores. Conidia of A. peruamazonensis are larger than A. chishuiensis and A. elliptica (28–36 × 17–21 µm vs. 23–29 × 14–21 µm vs. 14.5–18 × 9–10.5 µm), while conidiophores are shorter than the latter species (50–100 µm vs. 65–135 µm vs. 60–160 µm) (Matsushima 1993; Manoharachary et al. 2006). Molecular data is not available for A. peruamazonensis and A. elliptica.
Acrodictys effusa L.L. Liu, J. Yang & Z.Y. Liu, sp. nov.
Index Fungorum number: IF559868; Facesoffungi number: FoF12848; Fig. 99
Etymology: referring to the effuse colonies on natural substrates.
Holotype: GZAAS 20-0446
Saprobic on decaying submerged wood in freshwater habitats. Asexual morph: Colonies effuse, dark brown to black, hairy, scattered. Mycelium mostly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, unbranched, straight, 3–6-septate, dark brown at the base, slightly narrower and paler towards the apex, smooth-walled, thick-walled, 60–130 × 3.5–7 μm (\({\overline{\text{x}}}\) = 95 × 5 μm, n = 20). Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical, brown, smooth-walled. Conidia acrogenous, solitary, muriform, broadly obovoid to pyriform, with 3 transverse septa and a longitudinal septum, brown, paler in the basal cell, 18–24.5 × 12–16 μm (\({\overline{\text{x}}}\) = 21.5 × 13.5 μm, n = 20), guttulate, with conspicuous septal pores, slightly constricted at the septa, with a obconical basal cell and truncate base. Sexual morph: Undetermined.
Cultural characteristics: Conidia and conidiophores germinating on WA medium within 24 h. Colonies on PDA medium reaching 10–15 mm diam. in 3 weeks at 25 °C in dark, circular, with velvety, olivaceous brown mycelium on the surface, sparse at the edge; in reverse dark brown with entire margin.
Material examined: CHINA, Guizhou Province, Chishui City, 28.417° N, 106° E, on decaying wood submerged in a freshwater stream, 16 July 2019, L.L. Liu, CS1-1 (GZAAS 20-0446, holotype), ex-type culture GZCC 19-0551.
Notes: Acrodictys effusa is similar to A. aquatica (Hyde et al. 2018; Luo et al. 2019), A. atroapicula (Wang and Sutton 1982), A. elaeidicola (Ellis 1961), A. liputii (Cai et al. 2002c) and A. pyriformis (this study) in having brown, obovoid to pyriform, 3-transverse-septate conidia with similar dimension, and paler basal cells. Conidia with more than three transverse septa (3–4 or 3–5) are described in A. aquatica, A. atroapicula and the reference specimen of A. elaeidicola and A. liputii (Wang and Sutton 1982; Xia et al. 2017; Hyde et al. 2018). Acrodictys effusa, A. pyriformis, and the holotype of A. elaeidicola and A. liputii were observed with up to three transverse septa. Although the above species were described with brown conidia, they vary in their pigmentation. The phylogenetic analysis revealed A. effusa (GZCC 19-0551) grouped with a reference strain of A. bambusicola (HSAUPmyr 9510) as a sister taxon (Fig. 53). The LSU and ITS sequences of A. effusa (GZCC 19-0551) differs from A. bambusicola (HSAUPmyr 9510) by four bp (505/509 bp) and 15 bp (412/427 bp), respectively. Acrodictys bambusicola is distinguished from A. effusa by larger conidia (17–36 × 12–18 μm, holotype; 20–29 × 12.5–22.5 μm, reference specimen) with 2–5 transverse septa (Ellis 1961; Xia et al. 2017).
Acrodictys pyriformis J. Yang, Jian K. Liu & K.D. Hyde, sp. nov.
Index Fungorum number: IF559867; Facesoffungi number: FoF12847; Fig. 100
Etymology: referring to the pyriform conidia.
Holotype: HKAS 112613
Saprobic on decaying submerged wood in freshwater habitats. Asexaul morph: Colonies on wood effuse, hairy, dark brown, scattered, glistening. Mycelium mostly immersed, composed of septate, smooth-walled, brown to hyaline hyphae. Conidiophores macronematous, mononematous, erect, straight, or slightly curved, cylindrical, septate, smooth-walled, unbranched, brown to grayish brown, slightly paler and narrower towards the apex, 43–146 µm long, 3–7.5 µm wide near the base, 1.5–3 µm wide at the apex. Conidiogenous cells monoblastic, integrated, terminal, determinate, cylindrical to lageniform, brown, truncate at the apex, sometimes elongating percurrently. Conidia acrogenous, solitary, broadly obovoid to pyriform, muriform, usually with three transverse septa and a longitudinal septum, smooth-walled, pale brown to pale grayish green, becoming slightly darker with age, 20–30 × 12.5–21 µm (\({\overline{\text{x}}}\) = 24.5 × 15.5 µm, n = 20), guttulate, slightly constricted at the septa, with conspicuous septal pores, truncate at the base, basal cell obconical, pale brown to subhyaline. Sexual morph: Undetermined.
Culture characteristics: Conidia germinating on WA medium within 24 h and swollen germ tubes produced from the basal cell. Colonies on PDA medium reaching 10–15 mm in 1 month at 25 °C in dark, with dense mycelium on the surface, grayish brown in the middle, dark brown at the edge; in reverse dark brown with irregular margin.
Material examined: CHINA, Guizhou Province, Chishui City, Sidonggou Waterfall, 28.456° N, 105.648° E, on decaying twig submerged in a freshwater stream, 11 July 2019, J. Yang, CS31-1 (HKAS 112613, holotype; HKAS 125926, isotype), ex-type culture GZCC 20-0507; ibid, CS42-2-2 (HKAS 124632, paratype), ex-paratype culture GZCC 20-0514; CHINA, Guizhou Province, Chishui City, on submerged decaying twig, 16 July 2019, L.L. Liu, CS1-2-4 (GZAAS 20-0447, paratype), ex-paratype culture GZCC 19-0552.
Notes: Among Acrodictys species, A. aquatica (Hyde et al. 2018; Luo et al. 2019), A. atroapicula (Wang and Sutton 1982), A. bambusicola (Ellis 1961), A. elaeidicola (Ellis 1961), A. fluminicola (Luo et al. 2019), A. liputii (Cai et al. 2002c), A. porosiseptata (Zhao et al. 2011; Dong et al. 2021a), A. similis (Holubová-Jechová and Mercado-Sierra 1984) and two new species A. pyriformis and A. effusa share lageniform or cylindrical conidiogenous cells and obovoid to pyriform conidia with less than six transverse septa, mostly 1–2 (mostly one) longitudinal or oblique septa at upper cells and no vertical septa at the two basal cells. Their conidiophores are consistent in width but vary in length. They can be distinguished by conidial color, size, septa number and septal pores. Acrodictys pyriformis resembles the holotype of A. liputii by pale brown, mostly 3-tansverse-septate conidia with septal pores. However, A. pyriformis differs from A. liputii by shorter conidiophores (43–146 µm vs. 75–220 µm) and larger conidia (20–30 × 12.5–21 µm vs. 18.5–22.5 × 13.5–17.5 µm) (Cai et al. 2002c). The reference specimen of A. liputii differs by pale to reddish brown conidia with 3–4 transverse septa (Xia et al. 2017). Acrodictys pyriformis resembles A. effusa in conidial ontogeny and similar dimensions of conidiophores and conidia. However, A. effusa has darker conidia and is well distinguished by molecular DNA data (Fig. 53). Acrodictys pyriformis produces swollen germ tubes which are undescribed in other Acrodictys species.
In the phylogenetic analysis (Fig. 53), A. pyriformis (GZCC 20-0514, GZCC 20-0507 and GZCC 19-0552) clustered as sister to A. hainanensis (HSAUPmyr 7561). The LSU and ITS sequences of A. pyriformis (GZCC 20-0507, ex-type) differ from A. hainanensis (HSAUPmyr 7561) by three bp (505/508 bp) and seven bp (429/436 bp), respectively. Acrodictys hainanensis was described from a sporulating culture. It produces mid-brown, ellipsoidal to obovoid conidia with 3–5 transverse septa, several longitudinal or oblique septa and cylindrical, pale brown basal cells, which are remarkably different from A. pyriformis on natural substrates (Xia et al. 2017). Without comparable morphology and protein-coding genes, we prefer to recognize A. pyriformis as a distinct species from A. hainanensis. Further molecular evidence, cultural studies, and other specimens are needed to confirm the relationship between A. pyriformis and A. hainanensis.
Conclusion
This study detects the diversity and establishes the classification of freshwater fungi from karst regions in China (Guizhou Province) and Thailand based on morphological examinations and phylogenetic analyses. Freshwater fungi recorded in this study are mainly distributed in Dothideomycetes and Sordariomycetes, with a few in Eurotiomycetes. More than 90 species are well described and illustrated with half being new lineages. A large number of documented freshwater fungi in this study indicates high undiscovered diversity of freshwater fungi in the karst regions. A huge potential to study freshwater fungi’s taxonomy, ecology, and secondary metabolites is emerging. This discovery increases the fungal resources in China and Thailand and may gain the interest of more researchers to work on this fungal group.
We are indeed limited by the lack of molecular data of many old species that makes their classification unclear, and some similar species showed polyphyletic phylogeny. Therefore, it is recommended to epitypify or provide reference specimens for old species and resolve species complex. Holomorphic species are rarely described compared to species in only one state. The sexual-asexual connection needs to be clarified, and new methods to prompt culture sporulation should be developed. The present studies mainly focus on freshwater fungi in surface water. It is suggested to investigate freshwater fungi growing in groundwater from karst landscapes.
References
Abarca GH, Arias-Mota RM, Mena-Portales J, Mercado-Sierra A (2006) Adiciones al conocimiento de la diversidad de los hongos conidiales del bosque mesófilo de montaña del Estado de Veracruz. II. Acta Bot Mex 77:15–30. https://doi.org/10.21829/abm77.2006.1023
Abdel-Aziz FA (2016) The genus Lolia from freshwater habitats in Egypt with one new species. Phytotaxa 267:279–288. https://doi.org/10.11646/phytotaxa.267.4.4
Abdel-Aziz FA, Abdel-Wahab MA (2010) Lolia aquatica gen. et sp. nov. (Lindgomycetaceae, Pleosporales), a new coelomycete from freshwater habitats in Egypt. Mycotaxon 114:33–42. https://doi.org/10.5248/114.33
Abdel-Raheem A, Shearer CA (2002) Extracellular enzyme production by freshwater ascomycetes. Fungal Divers 11:1–19
Abdel-Wahab MA, Jones EBG (2000) Three new marine Ascomycetes from driftwood in Australia sand dunes. Mycoscience 41:379–388. https://doi.org/10.1007/BF02463951
Abdel-Wahab MA, Pang KL, Nagahama T, Abdel-Aziz FA, Jones EBG (2010) Phylogenetic evaluation of anamorphic species of Cirrenalia and Cumulospora with the description of eight new genera and four new species. Mycol Prog 9:537–558. https://doi.org/10.1007/s11557-010-0661-x
Arambarri AM, Cabello MN (1989) A numerical taxonomic study of some phialidic genera of hyphomycetes: cluster analysis. Mycotaxon 34:679–696
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Chukeatirote E, Kang JC, Xu JC, McKenzie EHC, Jones EBG, Hyde KD (2014) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Divers 68:69–104. https://doi.org/10.1007/s13225-014-0305-6
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen JJ, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBG, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel-Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal Forno M, Dollhofer V, Fliegerová K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytövuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Lumbsch HT, Matsumura M, Moncada B, Nuankaew S, Parnmen S, de Azevedo SALCM, Sommai S, Song Y, de Souza CAF, de Souza-Motta CM, Su HY, Suetrong S, Wang Y, Wei SF, Wen TC, Yuan HS, Zhou LW, Réblová M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH (2015a) Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:27–274. https://doi.org/10.1007/s13225-015-0346-5
Ariyawansa HA, Thambugala KM, Manamgoda DS, Jayawardena RS, Camporesi E, Boonmee S, Wanasinghe DN, Phookamsak R, Hongsanan S, Singtripop C, Chukeatirot E, Kang JC, Jones EBG, Hyde KD (2015b) Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers 71:85–139. https://doi.org/10.1007/s13225-015-0323-z
Arnaud G (1954) Mycologie concrète: genera II. Bull Trimest Soc Mycol Fr 69:265–306
Arzanlou M, Groenewald JZ, Gams W, Braun U, Crous PW (2007) Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Stud Mycol 58:57–93. https://doi.org/10.3114/sim.2007.58.03
Asthana A, Shearer CA (1990) Antagonistic activity of Pseudohalonectria and Ophioceras. Mycologia 82:554–561. https://doi.org/10.1080/00275514.1990.12025928
Atkinson TJ, Miller AN, Huhndorf SM, Orlovich DA (2007) Unusual new Chaetosphaeria species from New Zealand: intrafamilial diversity and elucidations of the Chaetosphaeriaceae-Lasiosphaeriaceae relationship (Sordariomycetes, Ascomycotina). N Z J Bot 45:685–706. https://doi.org/10.1080/00288250709509744
Badali H, Yazdanparast SA, Bonifaz A, Mousavi B, De Hoog GS, Klaassen CH, Meis JF (2013) Veronaea botryosa: molecular identification with amplified fragment length polymorphism (AFLP) and in vitro antifungal susceptibility. Mycopathologia 175:505–513. https://doi.org/10.1007/s11046-013-9631-6
Baker WA, Morgan-Jones G (2003) Notes on hyphomycetes XCI. Pseudoacrodictys, a novel genus for seven taxa formerly placed in Acrodictys. Mycotaxon 85:371–391
Baker WA, Partridge EC, Morgan-Jones G (2001) Notes on hyphomycetes LXXXI. Acrodictyella obovata, a new lignicolous, dematiaceous genus and species collected in Alabama. Mycotaxon 78:29–35
Baker WA, Partridge EC, Morgan-Jones G (2002a) Notes on hyphomycetes LXXXV. Junewangia, a genus in which to classify four Acrodictys species and a new taxon. Mycotaxon 81:293–319
Baker WA, Partridge EC, Morgan-Jones G (2002b) Notes on hyphomycetes LXXXVII. Rhexoacrodictys, a new segregate genus to accommodate four species previously classified in Acrodictys. Mycotaxon 82:95–113
Bao DF, Luo ZL, Liu JK, Bhat DJ, Sarunya N, Li WL, Su HY, Hyde KD (2018) Lignicolous freshwater fungi in China III: new species and record of Kirschsteiniothelia from northwestern Yunnan Province. Mycosphere 9:755–768. https://doi.org/10.5943/mycosphere/9/4/4
Bao DF, Luo ZL, Su XJ, Su HY (2021a) Hongkongmyces brunneisporus sp. Nov. (Lindgomycetaceae) from freshwater habitats in Yunnan Province and Tibet, Southwest China. Mycosystema 40:1274–1285. https://doi.org/10.13346/j.mycosystema.200307
Bao DF, Hyde KD, McKenzie EHC, Jeewon R, Su HY, Nalumpang S, Luo ZL (2021b) Biodiversity of lignicolous freshwater hyphomycetes from China and Thailand and description of sixteen species. J Fungi 7:669. https://doi.org/10.3390/jof7080669
Barr ME (1987) New taxa and combinations in the Loculoascomycetes. Mycotaxon 29:501–505
Barr ME (1990) Some dictyosporous genera and species of Pleosporales in North America. Mem N Y Bot Gard 62:1–92
Behnke-Borowczyk J, Kowalkowski W, Kartawik N, Baranowska M, Barzdajn W (2020) Soil fungal communities in nurseries producing Abies alba. Balt For 26:426. https://doi.org/10.46490/BF426
Berkeley MJ, Broome CE (1873) Enumeration of the fungi of Ceylon. Part II. J Linn Soc Bot 14:29–141
Bhat DJ, Kendrick B (1993) Twenty-five new conidial fungi from the Western Ghats and the Andaman Islands (India). Mycotaxon 49:19–90
Bhat DJ, Sutton BC (1985a) Some ‘phialidic’ hyphomycetes from Ethiopia. Trans Br Mycol Soc 84:723–730. https://doi.org/10.1016/S0007-1536(85)80130-3
Bhat DJ, Sutton BC (1985b) New and interesting hyphomycetes from Ethiopia. Trans Br Mycol Soc 85:107–122. https://doi.org/10.1016/S0007-1536(85)80160-1
Bizzozero G (1885) Funghi Veneti novi vel critici. Atti Dell’istituto Veneto Scienze 3:303–309
Boonmee S, KoKo TW, Chukeatirote E, Hyde KD, Chen H, Cai L, McKenzie EHC, Jones EBG, Kodsueb R, Hassan BA (2012) Two new Kirschsteiniothelia species with Dendryphiopsis anamorphs cluster in Kirschsteiniotheliaceae fam. nov. Mycologia 104:698–714. https://doi.org/10.3852/11-089
Boonmee S, Rossman AY, Liu JK, Li WJ, Dai DQ, Bhat DJ, Jones EBG, McKenzie EHC, Xu JC, Hyde KD (2014) Tubeufiales, ord. nov., integrating sexual and asexual generic names. Fungal Divers 68:239–298. https://doi.org/10.1007/s13225-014-0304-7
Boonmee S, D’souza MJ, Luo ZL, Pinruan U, Tanaka K, Su HY, Bhat DJ, McKenzie EHC, Jones EBG, Taylor JE, Phillips AJL, Hirayama K, Eungwanichayapant PD, Hyde KD (2016) Dictyosporiaceae fam. nov. Fungal Divers 80:457–482. https://doi.org/10.1007/s13225-016-0363-z
Boonmee S, Wanasinghe DN, Calabon MS, Huanraluek N, Chandrasiri SKU, Jones EBG, Rossi W, Leonardi M, Singh SK, Rana S, Singh PN, Maurya DK, Lagashetti AC, Choudhary D, Dai YC, Zhao CL, Mu YH, Yuan HS, He SH, Phookamsak R, Jiang HB, Martín MP, Dueñas M, Telleria MT, Kałucka IL, Jagodziński AM, Liimatainen K, Pereira DS, Phillips AJL, Suwannarach N, Kumla J, Khuna S, Lumyong S, Potter TB, Shivas RG, Sparks AH, Vaghefi N, Abdel-Wahab MA, Abdel-Aziz FA, Li GJ, Lin WF, Singh U, Bhatt RP, Lee HB, Nguyen TTT, Kirk PM, Dutta AK, Acharya K, Sarma VV, Niranjan M, Rajeshkumar KC, Ashtekar N, Lad S, Wijayawardene NN, Bhat DJ, Xu RJ, Wijesinghe SN, Shen HW, Luo ZL, Zhang JY, Sysouphanthong P, Thongklang N, Bao DF, Aluthmuhandiram JVS, Abdollahzadeh J, Javadi A, Dovana F, Usman M, Khalid AN, Dissanayake AJ, Telagathoti A, Probst M, Peintner U, Garrido-Benavent I, Bóna L, Merényi Z, Boros L, Zoltán B, Stielow JB, Jiang N, Tian CM, Shams E, Dehghanizadeh F, Pordel A, Javan-Nikkhah M, Denchev TT, Denchev CM, Kemler M, Begerow D, Deng CY, Harrower E, Bozorov T, Kholmuradova T, Gafforov Y, Abdurazakov A, Xu JC, Mortimer PE, Ren GC, Jeewon R, Maharachchikumbura SSN, Phukhamsakda C, Mapook A, Hyde KD (2021) Fungal diversity notes 1387–1511: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 111:1–335. https://doi.org/10.1007/s13225-021-00489-3
Boonyuen N, Chuaseeharonnachai C, Suetrong S, Sri-indrasutdhi V, Sivichai S, Jones EBG, Pang KL (2011) Savoryellales (Hypocreomycetidae, Sordariomycetes): a novel lineage of aquatic ascomycetes inferred from multiple-gene phylogenies of the genera Ascotaiwania, Ascothailandia, and Savoryella. Mycologia 103(6):1351–1371. https://doi.org/10.3852/11-102
Boonyuen N, Chuaseeharonnachai C, Suetrong S, Sujinda S, Somrithipol S (2016) Parafuscosporella garethii sp. nov. (Fuscosporellales) from a rivulet in a community-based northern forest, in Thailand. Mycosphere 7:1265–1272. https://doi.org/10.5943/mycosphere/7/9/2
Brahmanage RS, Dayarathne MC, Wanasinghe DN, Thambugala KM, Jeewon R, Chethana KWT, Samarakoon MC, Tennakoon DS, De Silva NI, Camporesi E, Raza M, Yan JY, Hyde KD (2020) Taxonomic novelties of saprobic Pleosporales from selected dicotyledons and grasses. Mycosphere 11:2481–2541. https://doi.org/10.5943/mycosphere/11/1/15
Cai L, Tsui CKM, Zhang KQ, Hyde KD (2002a) Aquatic fungi from Lake Fuxian, Yunnan, China. Fungal Divers 9:57–70
Cai L, Lumyong P, Zhang K, Hyde KD (2002b) New species of Annulatascus and Saccardoella from the Philippines. Mycotaxon 84:255–263
Cai L, Zhang K, McKenzie EHC, Ho WH, Hyde KD (2002c) Acrodictys liputii sp. nov. and Digitodesmium bambusicola sp. nov. from bamboo submerged in Liput River in the Philippines. Nova Hedwig 75:525–532. https://doi.org/10.1127/0029-5035/2002/0075-0525
Cai L, Zhang KQ, McKenzie EHC, Hyde KD (2003a) Freshwater fungi from bamboo and wood submerged in the Liput River in the Philippines. Fungal Divers 13:1–12
Cai L, Zhang K, McKenzi EHC, Hyde KD (2003b) New species of Dictyosporium and Digitodesmium from submerged wood in Yannan, China. Sydowia 55:129–135
Cai L, Jeewon R, Hyde KD (2005) Phylogenetic evaluation and taxonomic revision of Schizothecium based on ribosomal DNA and protein coding genes. Fungal Divers 19:1–21
Cai L, Hyde KD, Tsui CKM (2006) Genera of freshwater fungi. Fungal Divers Res Ser 18:1–261
Calabon MS, Hyde KD, Jones EBG, Doilom M, Liao CF (2020a) Mycoenterolobium aquadictyosporium sp. nov. (Pleosporomycetidae, Dothideomycetes) from a freshwater habitat in Thailand. Mycol Prog 19:1031–1042. https://doi.org/10.1007/s11557-020-01609-0
Calabon MS, Hyde KD, Jones EBG, Chandrasiri S, Dong W, Fryar SC, Yang J, Luo ZL, Lu YZ, Bao DF, Boonmee S (2020b) www. freshwaterfungi.org, an online platform for the taxonomic classification of freshwater fungi. Asian J Mycol 3:419–445. https://doi.org/10.5943/ajom/3/1/14
Calabon MS, Jones EBG, Hyde KD, Boonmee S, Tibell S, Tibell L, Pang KL, Phookamsak R (2021) Phylogenetic assessment and taxonomic revision of Halobyssothecium and Lentithecium (Lentitheciaceae, Pleosporales). Mycol Prog 20:701–720. https://doi.org/10.1007/s11557-021-01692-x
Calabon MS, Hyde KD, Jones EBG, Luo ZL, Dong W, Hurdeal VG, Gentekaki E, Rossi W, Leonardi M, Thiyagaraja V, Lestari AS, Shen HW, Bao DF, Boonyuen N, Zeng M (2022) Freshwater fungal numbers. Fungal Divers 114:3–235. https://doi.org/10.1007/s13225-022-00503-2
Campbell J, Shearer CA (2004) Annulusmagnus and Ascitendus, two new genera in the Annulatascaceae. Mycologia 96:822–833. https://doi.org/10.1080/15572536.2005.11832929
Castañeda-Ruíz RF (1988) Fungi Cubenses III. Instituto de Investigaciones Fundamentales en Agricultura Tropical. Alejandro de Humboldt
Castañeda-Ruiz RF, Arnold GRW (1985) Deuteromycotina de Cuba. I. Hyphomycetes. Rev Jard Bot Nac 6:47–67
Castañeda-Ruiz RF, Cano J, Guarro J (1997) Notes on conidial fungi VI. Menisporopsis. Mycotaxon 64:335–342
Catania M, Romero AI, Huhndorf SM, Miller AN (2011) A new species and new records of Cercophora from Argentina. Mycologia 103(6):1372–1383. https://doi.org/10.3852/11-005
Cesati V, de Notaris G (1863) Schema di classificazione degli sferiacei italici aschigeri più o meno appartenenti al genere Sphaeria nell’antico significato attribuitoglda Persoon. Comment Soc Crittogamia Ital 1:177–240
Chadefaud M (1960) Les végétaux non vasculaires: cryptogamie (Traité de botanique). Masson, Paris
Chang HS (1995) Notes on Taiwan dematiaceous hyphomycetes, some species of the genera Exserticlava, Craspedodidymum and Hermatomyces. Bot Bull Acad Sin 36:243–246
Chang H (2001) Trichocladium anamorph of Ascotaiwania hsilio and Monodictys-like anamorphic states of Ascotaiwania lignicola. Fungal Sci 16:35–38
Chang HS, Hsieh SY, Jones EBG, Read SJ, Moss ST (1998) New freshwater species of Ascotaiwania and Savoryella from Taiwan. Mycol Res 102:709–718. https://doi.org/10.1017/S0953756297005637
Chen YY, Dissanayake AJ, Liu ZY, Liu JK (2020a) Additions to Karst Fungi 4: Botryosphaeria spp. associated with woody hosts in Guizhou province, China including B. guttulata sp. nov. Phytotaxa 454:186–202. https://doi.org/10.11646/phytotaxa.454.3.2
Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, Xia R (2020b) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13(8):1194–1202. https://doi.org/10.1016/j.molp.2020.06.009
Chen YY, Dissanayake AJ, Liu JK (2021) Additions to Karst Fungi 5: Sardiniella guizhouensis sp. Nov. (Botryosphaeriaceae) associated with woody hosts in Guizhou province, China. Phytotaxa 508:187–196. https://doi.org/10.11646/phytotaxa.508.2.7
Chethana KWT, Manawasinghe IS, Hurdeal VG, Bhunjun CS, Appadoo MA, Gentekaki E, Olivier R, Promputtha I, Hyde KD (2021) What are fungal species and how to delineate them? Fungal Divers 109:1–25. https://doi.org/10.1007/s13225-021-00483-9
Chuaseeharonnachai C, Somrithipol S, Suetrong S, Klaysuban A, Pornputtapong N, Jones EBG, Boonyuena N (2017) Conioscypha nakagirii, a new species from naturally submerged wood in Thailand based on morphological and molecular data. Mycoscience 58:424–431. https://doi.org/10.1016/j.myc.2017.06.003
Cifferi R, Montemartini A (1958) Sui generi Muchmoria Sacc. e Veronaea n. gen. (Dematiaceae, Didymosporae). Atti1st Bot Univ Lab Crittogam, Pavia Ser 5 15:67–72
Cooper JA (2005) New Zealand hyphomycete fungi: additional records, new species, and notes on interesting collections. N Z J Bot 43:323–349. https://doi.org/10.1080/0028825X.2005.9512957
Corda ACI (1837) Icones fungorum. J.G. Calve, Pragae
Costa PMO, Malosso E, Barbosa MA, Tavares WL, Castañeda-Ruiz RF (2017) Minimelanolocus atlanticus sp. nov. and M. navicularis from the Brazilian Atlantic Forest. Mycotaxon 132:925–931. https://doi.org/10.5248/132.925
Crane JL, Shearer CA, Barr ME (1998) A revision of Boerlagiomyces with notes and a key to the saprobic genera of Tubeufiaceae. Can J Bot 76:602–612. https://doi.org/10.1139/b98-024
Cribb AB, Cribb JW (1960) Marine fungi from Queensland—III. Pap Dept Bot Univ Qd 4(3):40–44
Crous PW, Summerell BA, Shivas RG, Romberg M, Mel’nik VA, Verkley GJM, Groenewald JZ (2011) Fungal planet description sheets: 92–106. Persoonia 27:130–162. https://doi.org/10.3767/003158511X617561
Crous PW, Summerell BA, Shivas RG, Burgess TI, Decock CA, Dreyer LL, Granke LL, Guest DI, Hardy GESJ, Hausbeck MK, Hüberli D, Jung T, Koukol O, Lennox CL, Liew ECY, Lombard L, McTaggart AR, Pryke JS, Roets F, Saude C, Shuttleworth LA, Stukely MJC, Vánky K, Webster BJ, Windstam ST, Groenewald JZ (2012) Fungal Planet description sheets: 107–127. Persoonia 28:138–182. https://doi.org/10.3767/003158512X652633
Crous PW, Wingfield MJ, Guarro J, Cheewangkoon R, van der Bank M, Swart WJ, Stchigel AM, Cano-Lira JF, Roux J, Madrid H, Damm U, Wood AR, Shuttleworth LA, Hodges CS, Munster M, de Jesús Y-M, Zúñiga-Estrada L, Cruywagen EM, De Hoog GS, Silvera C, Najafzadeh J, Davison EM, Davison PJN, Barrett MD, Barrett RL, Manamgoda DS, Minnis AM, Kleczewski NM, Flory SL, Castlebury LA, Clay K, Hyde KD, Maússe-Sitoe SND, Chen S, Lechat C, Hairaud M, Lesage-Meessen L, Pawłowska J, Wilk M, Śliwińska-Wyrzychowska A, Mętrak M, Wrzosek M, Pavlic-Zupanc D, Maleme HM, Slippers B, Mac Cormack WP, Archuby DI, Grünwald NJ, Tellería MT, Dueñas M, Martín MP, Marincowitz S, de Beer ZW, Perez CA, Gené J, Marin-Felix Y, Groenewald JZ (2013) Fungal Planet description sheets: 154–213. Persoonia 31:188–296. https://doi.org/10.3767/003158513X675925
Crous PW, Wingfield MJ, Guarro J, Hernández-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Dueñas M, Dutta AK, Gené J, Gouliamova DE, Groenewald M, Lombard L, Morozova OV, Sarkar J, Smith MTH, Stchigel AM, Wiederhold NP, Alexandrova AV, Antelmi I, Armengol J, Barnes I, Cano-Lira JF, Ruiz RF, Castañeda Contu M, Courtecuisse PR, da Silveira AL, Decock CA, de Goes A, Edathodu J, Ercole E, Firmino AC, Fourie A, Fournier J, Furtado EL, Geering ADW, Gershenzon J, Giraldo A, Gramaje D, Hammerbacher A, He XL, Haryadi D, Khemmuk W, Kovalenko AE, Krawczynski R, Laich F, Lechat C, Lopes UP, Madrid H, Malysheva EF, Marín-Felix Y, Martín MP, Mostert L, Nigro F, Pereira OL, Picillo B, Pinho DB, Popov ES, Peláez CA, Rodas Rooney-Latham S, SandovalDenis M, Shivas RG, Silva V, Stoilova-Disheva MM, Telleria MT, Ullah C, Unsicker SB, van der Merwe NA, Vizzini A, Wagner HG, Wong PTW, Wood AR, Groenewald JZ (2015a) Fungal Planet description sheets: 320–370. Persoonia 34:167–266. https://doi.org/10.3767/003158515X688433
Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernández-Restrepo M, Jaklitsch WM, Lebrun MH, Schumacher RK, Stielow JB, van der Linde EJ, Vilcāne J, Voglmayr H, Wood AR (2015b) The Genera of Fungi—fixing the application of the type species of generic names—G2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 6:163–198. https://doi.org/10.5598/imafungus.2015.06.01.11
Crous PW, Schumacher RK, Wingfield MJ, Lombard L, Giraldo A, Christensen M, Gardiennet A, Nakashima C, Pereira O, Smith AJ, Groenewald JZ (2015c) Fungal systematics and evolution: FUSE 1. Sydowia 67:81–118. https://doi.org/10.12905/0380.sydowia67-2015c-0081
Crous PW, Wingfield MJ, Burgess TI, Hardy GESJ, Crane C, Barrett S, Cano-Lira JF, Le Roux JJ, Thangavel R, Guarro J, Stchigel AM, Martín MP, Alfredo DS, Barber PA, Barreto RW, Baseia IG, Cano-Canals J, Cheewangkoon R, Ferreira RJ, Gené J, Lechat C, Moreno G, Roets F, Shivas RG, Sousa JO, Tan YP, Wiederhold NP, Abell SE, Accioly T, Albizu JL, Alves JL, Antoniolli ZI, Aplin N, Araújo J, Arzanlou M, Bezerra JDP, Bouchara J-P, Carlavilla JR, Castillo A, Castroagudín VL, Ceresini PC, Claridge GF, Coelho G, Coimbra VRM, Costa LA, da Cunha KC, da Silva SS, Daniel R, de Beer ZW, Dueñas M, Edwards J, Enwistle P, Fiuza PO, Fournier J, García D, Gibertoni TB, Giraud S, Guevara-Suárez M, Gusmão LFP, Haituk S, Heykoop M, Hirooka Y, Hofmann TA, Houbraken J, Hughes DP, Kautmanová I, Koppel O, Koukol O, Larsson E, Latha KPD, Lee DH, Lisboa DO, Lisboa WS, López-Villalba Á, Maciel JLN, Manimohan P, Manjón JL, Marincowitz S, Marney TS, Meijer M, Miller AN, Olariaga I, Paiva LM, Piepenbring M, Poveda-Molero JC, Raj KNA, Raja HA, Rougeron A, Salcedo I, Samadi R, Santos TAB, Scarlett K, Seifert KA, Shuttleworth LA, Silva GA, Silva M, Siqueira JPZ, Souza-Motta CM, Stephenson SL, Sutton DA, Tamakeaw N, Telleria MT, Valenzuela-Lopez N, Viljoen A, Visagie CM, Vizzini A, Wartchow F, Wingfield BD, Yurchenko E, Zamora JC, Groenewald JZ (2016) Fungal planet description sheets: 469–557. Persoonia 37:218–403. https://doi.org/10.3767/003158516X694499
Crous PW, Wingfield MJ, Burgess TI, Carnegie AJ, Hardy GESJ, Smith D, Summerell BA, Cano-Lira JF, Guarro J, Houbraken J, Lombard L, Martín MP, Sandoval-Denis M, Alexandrova A, Barnes CW, Baseia IG, Bezerra JDP, Guarnaccia V, May TW, Hernández-Restrepo M, Stchige AM, Miller AN, Ordoñez ME, Abreu VP, Accioly T, Agnello C, Colmán AA, Albuquerque CC, Alfredo DS, Alvarado P, Araújo-Magalhães GR, Arauzo S, Atkinson T, Barili A, Barreto RW, Bezerra JL, Cabral TS, Rodríguez FC, Cruz RHSF, Daniëls PP, da Silva BDB, de Almeida DAC, Carvalho Júnior AA, Decock CA, Delgat L, Denman S, Dimitrov RA, Edwards J, Fedosova AG, Ferreira RJ, Firmino AL, Flores JA, García D, Gené J, Giraldo A, Góis JS, Gomes AAM, Gonçalves CM, Gouliamova DE, Groenewald M, Guéorguiev BV, Guevara-Suarez M, Gusmão LFP, Hosaka K, Hubka V, Huhndorf SM, Jadan M, Jurjević Ž, Kraak B, Kučera V, Kumar TKA, Kušan I, Lacerda SR, Lamlertthon S, Lisboa WS, Loizides M, Luangsa-ard JJ, Lysková P, Mac Cormack WP, Macedo DM, Machado AR, Malysheva EF, Marinho P, Matočec N, Meijer M, Mešić A, Mongkolsamrit S, Moreira KA, Morozova OV, Nair KU, Nakamura N, Noisripoom W, Olariaga I, Oliveira RJV, Paiva LM, Pawar P, Pereira OL, Peterson SW, Prieto M, RodríguezAndrade E, Blas CRD, Roy M, Santos ES, Sharma R, Silva GA, Souza-Motta CM, Takeuchi-Kaneko Y, Tanaka C, Thakur A, Smith MT, Tkalčec Z, Valenzuela-Lopez N, van der Kleij P, Verbeken A, Viana MG, Wang XW, Groenewald JZ (2017) Fungal planet description sheets: 625–715. Persoonia 39:270–467. https://doi.org/10.3767/persoonia.2017.39.11
Crous PW, Schumacher RK, Wingfield MJ, Akulov A, Denman S, Roux J, Braun U, Burgess TI, Carnegie AJ, Váczy KZ, Guatimosim E, Schwartsburd PB, Barreto RW, Hernández-Restrepo M, Lombard L, Groenewald JZ (2018a) New and interesting fungi. 1. Fungal Syst Evol 1:169–215. https://doi.org/10.3114/fuse.2018.01.08
Crous PW, Wingfield MJ, Burgess TI, Hardy GESJ, Gené J, Guarro J, Baseia IG, García D, Gusmão LFP, Souza-Motta CM, Thangavel R, Adamčík S, Barili A, Barnes CW, Bezerra JDP, Bordallo JJ, Cano-Lira JF, de Oliveira RJV, Ercole E, Hubka V, IturrietaGonzález I, Kubátová A, Martín MP, Moreau PA, Morte A, Ordoñez ME, Rodríguez A, Stchigel AM, Vizzini A, Abdollahzadeh J, Abreu VP, Adamčíková K, Albuquerque GMR, Alexandrova AV, Álvarez Duarte E, Armstrong-Cho C, Banniza S, Barbosa RN, Bellanger JM, Bezerra JL, Cabral TS, Caboň M, Caicedo E, Cantillo T, Carnegie AJ, Carmo LT, CastañedaRuiz RF, Clement CR, Čmoková A, Conceição LB, Cruz RHSF, Damm U, da Silva BDB, da Silva GA, da Silva RMF, Santiago ALCMdA, de Oliveira LF, de Souza CAF, Déniel F, Dima B, Dong G, Edwards J, Félix CR, Fournier J, Gibertoni TB, Hosaka K, Iturriaga T, Jadan M, Jany JL, Jurjević Ž, Kolařík M, Kušan I, Landell MF, Leite Cordeiro TR, Lima DX, Loizides M, Luo S, Machado AR, Madrid H, Magalhães OMC, Marinho P, Matočec N, Mešić A, Miller AN, Morozova OV, Neves RP, Nonaka K, Nováková A, Oberlies NH, Oliveira-Filho JRC, Oliveira TGL, Papp V, Pereira OL, Perrone G, Peterson SW, Pham THG, Raja HA (2018b) Fungal Planet description sheets: 716–784. Persoonia 40:239–392. https://doi.org/10.3767/persoonia.2018.40.10
Crous PW, Luangsa-Ard JJ, Wingfield MJ, Carnegie AJ, HernándezRestrepo M, Lombard L, Roux J, Barreto RW, Baseia IG, CanoLira JF, Martín MP, Morozova OV, Stchigel AM, Summerell BA, Brandrud TE, Dima B, García D, Giraldo A, Guarro J, Gusmão LFP, Khamsuntorn P, Noordeloos ME, Nuankaew S, Pinruan U, Rodríguez-Andrade E, Souza-Motta CM, Thangavel R, van Iperen AL, Abreu VP, Accioly T, Alves JL, Andrade JP, Bahram M, Baral HO, Barbier E, Barnes CW, Bendiksen E, Bernard E, Bezerra JDP, Bezerra JL, Bizio E, Blair JE, Bulyonkova TM, Cabral TS, Caiafa MV, Cantillo T, Colmán AA, Conceição LB, Cruz S, Cunha AOB, Darveaux BA, da Silva AL, da Silva GA, da Silva GM, da Silva RMF, de Oliveira RJV, Oliveira RL, De Souza JT, Dueñas M, Evans HC, Epifani F, Felipe MTC, Fernández-López J, Ferreira BW, Figueiredo CN, Filippova NV, Flores JA, Gené J, Ghorbani G, Gibertoni TB, Glushakova AM, Healy R, Huhndorf SM, Iturrieta-González I, Javan-Nikkhah M, Juciano RF, Jurjević Ž, Kachalkin AV, Keochanpheng K, Krisai-Greilhuber I, Li YC, Lima AA, Machado AR, Madrid H, Magalhães OMC, Marbach PAS, Melanda GCS, Miller AN, Mongkolsamrit S, Nascimento RP, Oliveira TGL, Ordoñez ME, Orzes R, Palma MA, Pearce CJ, Pereira OL, Perrone G, Peterson SW, Pham THG, Piontelli E, Pordel A, Quijada L, Raja HA, Rosas de Paz E, Ryvarden L, Saitta A, Salcedo SS, SandovalDenis M, Santos TAB, Seifert KA, Silva BDB, Smith ME, Soares AM, Sommai S, Sousa JO, Suetrong S, Susca A, Tedersoo L, Telleria MT, Thanakitpipattana D, Valenzuela-Lopez N, Visagie CM, Zapata M, Groenewald JZ (2018c) Fungal Planet description sheets: 785–867. Persoonia 41:238–417. https://doi.org/10.3767/persoonia.2018.41.12
Crous PW, Schumacher RK, Wood AR, Groenewald JZ (2020a) The Genera of Fungi—G5: Arthrinium, Ceratosphaeria, Dimerosporiopsis, Hormodochis, Lecanostictopsis, Lembosina, Neomelanconium, Phragmotrichum, Pseudomelanconium, Rutola, and Trullula. Fungal Syst Evol 5:77–98. https://doi.org/10.3114/fuse.2020.05.04
Crous PW, Wingfield MJ, Chooi YH, Gilchrist CLM, Lacey E, Pitt JI, Roets F, Swart WJ, Cano-Lira JF, Valenzuela-Lopez N, Hubka V, Shivas RG, Stchigel AM, Holdom DG, Jurjević Z, Kachalkin AV, Lebel T, Lock C, Martín MP, Tan YP, Tomashevskaya MA, Vitelli JS, Baseia IG, Bhatt VK, Brandrud TE, De Souza JT, Dima B, Lacey HJ, Lombard L, Johnston PR, Morte A, Papp V, Rodríguez A, Rodríguez-Andrade E, Semwa KC, Tegart L, Abad ZG, Akulov A, Alvarado P, Alves A, Andrade JP, Arenas F, Asenjo C, Ballarà J, Barrett MD, Berná LM, Berraf-Tebba A, Bianchinotti MV, Bransgrove K, Burgess TI, Carmo FS, Chávez R, Čmoková A, Dearnaley JDW, de Santiago ALCM, Freitas-Neto JF, Denman S, Douglas B, Dovana F, Eichmeier A, Esteve-Raventós F, Farid A, Fedosova AG, Ferisin G, Ferreira RJ, Ferrer A, Figueiredo CN, Figueiredo YF, ReinosoFuentealba CG, Garrido-Benavent I, Cañete-Gibas CF, Gil-Durán C, Glushakova AM, Gonçalves MFM, González M, Gorczak M, Gorton C, Guard FE, Guarnizo AL, Guarro J, Gutiérrez M, Hama P, Hien LT, Hocking AD, Houbraken J, Hunter GC, Inácio CA, Jourdan M, Kapitonov VI, Kelly L, Khan TN, Kisło K, Kiss L, Kiyashko A, Kolařík M, Kruse J, Kubátová A, Kučera V, Kučerová I, Kušan I, Lee HB, Levicán G, Lewis A, Liem NV, Liimatainen K, Lim HJ, Lyons MN, Maciá-Vicente JG, MagañaDueñas V, Mahiques R, Malysheva EF, Marbach PAS, Marinho P, Matočec N, McTaggart AR, Mešić A, Morin L, Muñoz-Mohedano JM, Navarro-Ródenas A, Nicolli CP, Oliveira RL, Otsing E, Ovrebo CL, Pankratov TA, Paños A, Paz-Conde A, Pérez-Sierra A, Phosri C, Pintos Á, Pošta A, Prencipe S, Rubio E, Saitta A, Sales LS, Sanhueza L, Shuttleworth LA, Smith J, Smith ME, Spadaro D, Spetik M, Sochor M, Sochorová Z, Sousa JO, Suwannasai N, Tedersoo L, Thanh HM, Thao LD, Tkalčec Z, Vaghefi N, Venzhik AS, Verbeken A, Vizzini A, Voyron S, Wainhouse M, Whalley AJS, Wrzosek M, Zapata M, Zeil-Rolfe I, Groenewald JZ (2020b) Fungal planet description sheets: 1042–1111. Persoonia 44:301–459. https://doi.org/10.3767/persoonia.2020.44.11
Crous PW, Osieck ER, Jurjevi Ž, Boers J, Van Iperen AL, Starink-Willemse M, Dima B, Balashov S, Bulgakov TS, Johnston PR, Morozova OV, Pinruan U, Sommai S, Alvarado P, Decock CA, Lebel T, McMullan-Fisher S, Moreno G, Shivas RG, Zhao L, Abdollahzadeh J, Abrinbana M, Ageev DV, Akhmetova G, Alexandrova AV, Altés A, Amaral AGG, Angelini C, Antonín V, Arenas F, Asselman P, Badali F, Baghela A, Bañares A, Barreto RW, Baseia IG, Bellanger JM, Berraf-Tebbal A, Yu BA, Bukharova NV, Burgess TI, Cabero J, Câmara MPS, Cano-Lira JF, Ceryngier P, Chávez R, Cowan DA, de Lima AF, Oliveira RL, Denman S, Dang QN, Dovana F, Duarte IG, Eichmeier A, Erhard A, Esteve-Raventós F, Fellin A, Ferisin G, Ferreira RJ, Ferrer A, Finy P, Gaya E, Geering ADW, Gil-Durán C, Glässnerová K, Glushakova AM, Gramaje D, Guard FE, Guarnizo AL, Haelewaters D, Halling RE, Hill R, Hirooka Y, Hubka V, Iliushin VA, Ivanova DD, Ivanushkina NE, Jangsantear P, Justo A, Kachalkin AV, Kato S, Khamsuntorn P, Kirtsideli IY, Knapp DG, Kochkina GA, Koukol O, Kovács GM, Kruse J, Kumar TKA, Kušan I, Læssøe T, Larsson E, Lebeuf R, Levicán G, Loizides M, Marinho P, Luangsa-ard JJ, Lukina EG, Magaña-Dueñas V, Maggs-Kölling G, Malysheva EF, Malysheva VF, Martín B, Martín MP, Matočec N, McTaggart AR, Mehrabi-Koushki M, Mešić A, Miller AN, Mironova P, Moreau PA, Morte A, Müller K, Nagy LG, Nanu S, Navarro-Ródenas A, Nel WJ, Nguyen TH, Nóbrega TF, Noordeloos ME, Olariaga I, Overton BE, Ozerskaya SM, Palani P, Pancorbo F, Papp V, Pawłowska J, Pham TQ, Phosri C, Popov ES, Portugal A, Pošta A, Reschke K, Reul M, Ricci GM, Rodríguez A, Romanowski J, Ruchikachorn N, Saar I, Safi A, Sakolrak B, Salzmann F, Sandoval-Denis M, Sangwichein E, Sanhueza L, Sato T, Sastoque A, Senn-Irlet B, Shibata A, Siepe K, Somrithipol S, Spetik M, Sridhar P, Stchigel AM, Stuskova K, Suwannasai N, Tan YP, Thangavel R, Tiago I, Tiwari S, Tkalčec Z, Tomashevskaya MA, Tonegawa C, Tran HX, Tran NT, Trovão J, Trubitsyn VE, Van Wyk J, Vieira WAS, Vila J, Visagie CM, Vizzini A, Volobuev SV, Vu DT, Wangsawat N, Yaguchi T, Ercole E, Ferreira BW, de Souza AP, Vieira BS, Groenewald JZ (2021) Fungal Planet description sheets: 1284–1382. Persoonia 47:178–374. https://doi.org/10.3767/persoonia.2021.47.06
Cruz ACR, Marques MFO, Gusmão LFP (2014) Conidial fungi from the semi-arid Caatinga biome of Brazil: the genus Menisporopsis. Acta Bot Bras 28:339–345. https://doi.org/10.1590/0102-33062014abb3207
Czeczuga B, Orłowska M (1999) Hyphomycetes in rainwater, melting snow and ice. Acta Mycol 34:181–200. https://doi.org/10.5586/am.1999.014
Czeczuga B, Orłowska M (2001) Hyphomycetes species on floating plant spores and pollen. Acta Hydrochim Hydrobiol 29:100–110. https://doi.org/10.1002/1521-401X(200109)29:2/3%3c100::AID-AHEH100%3e3.0.CO;2-9
Czeczuga B, Kiziewicz B, Mazalska B (2003) Further studies on aquatic fungi in the river Biebrza within Biebrza National Park. Pol J Environ Stud 12(5):531–543
Dai DQ, Bahkali AH, Ariyawansa HA, Li WJ, Bhat DJ, Hyde KD (2016) Neokalmusia didymospora (Didymosphaeriaceae), a new species from bamboo. Sydowia 68:17–25. https://doi.org/10.12905/0380.sydowia68-2016-0017
Dai DQ, Tang LZ, Liu C, Wang HB, Hyde KD (2018) Studies on Parmulariaceae I. A phylogeny based on available sequence data; introducing Parmulariales ord. nov., and Hemigraphaceae, Melaspileellaceae and Stictographaceae fam. nov. Phytotaxa 369(2):63–79. https://doi.org/10.11646/phytotaxa.369.2.1
Dayarathne MC, Maharachchikumbura SSN, Phookamsak R, Fryar SC, To-anun C, Jones EBG, Al-Sadi AM, Zelski SE, Hyde KD (2016) Morpho-molecular characterization and epitypification of Annulatascus velatisporus. Mycosphere 7(9):1389–1398. https://doi.org/10.5943/mycosphere/7/9/12
Dayarathne MC, Wanasinghe DN, Jones EBG, Chomnunti P, Hyde KD (2018) A novel marine genus Halobyssothecium (Lentitheciaceae) and epitypification of Halobyssothecium obiones comb. nov. Mycol Prog 17:1161–1171. https://doi.org/10.1007/s11557-018-1432-3
Dayarathne MC, Maharachchikumbura SSN, Jones EBG, Dong W, Devadatha B, Yang J, Ekanayake AH, De Silva W, Sarma VV, Al-Sadi AM, Khongphinitbunjong K, Hyde KD, Zhao RL (2019) Phylogenetic revision of Savoryellaceae and evidence for its ranking as a subclass. Front Microbiol 10:840. https://doi.org/10.3389/fmicb.2019.00840
Dayarathne MC, Jones EBG, Maharachchikumbura SSN, Devadatha B, Sarma VV, Khongphinitbunjong K, Chomnunti P, Hyde KD (2020) Morpho-molecular characterization of microfungi associated with marine based habitats. Mycosphere 11:1–188. https://doi.org/10.5943/mycosphere/11/1/1
de Hoog GS (2014) Ecology and phylogeny of black yeast-like fungi: diversity in unexplored habitats. Fungal Divers 65:1–2. https://doi.org/10.1007/s13225-014-0284-7
de Hoog GS, Papendorf MC (1976) The genus Phaeoisaria. Persoonia 8(4):407–414
de Notaris G (1857) Micromicetes italici novi vel minus cogniti, decas 9. Memorie Della Reale Accademia Delle Scienze Di Torino 16:457–471
de Waele J, Plan L, Audra P (2009) Recent developments in surface and subsurface karst geomorphology: an introduction. Geomorphology 106:1–8. https://doi.org/10.1016/j.geomorph.2008.09.023
Delgado G, Koukol O, Cáceres O, Piepenbring M (2017) The Phylogenetic placement of Ernakulamia cochinensis within Pleosporales (Dothideomycetes, Ascomycota). Cryptogam Mycol 38(4):435–451. https://doi.org/10.7872/crym/v38.iss4.2017.435
Devadatha B, Calabon MS, Abeywickrama PD, Hyde KD, Jones EBG (2020) Molecular data reveals a new holomorphic marine fungus, Halobyssothecium estuariae, and the asexual morph of Keissleriella phragmiticola. Mycology 11:167–183. https://doi.org/10.1080/21501203.2019.1700025
DiCosmo F, Berch S, Kendrick WB (1983) Cylindrotrichum, Chaetopsis, and two new genera of hyphomycetes, Kylindria and Xenokylindria. Mycologia 75:949–973. https://doi.org/10.1080/00275514.1983.12023781
Diederich P, Ertz D, Lawrey JD, Sikaroodi M, Untereiner WA (2013) Molecular data place the hyphomycetous lichenicolous genus Sclerococcum close to Dactylospora (Eurotiomycetes) and S. parmeliae in Cladophialophora (Chaetothyriales). Fungal Divers 58:61–72. https://doi.org/10.1007/s13225-012-0179-4
Diederich P, Lawrey JD, Ertz D (2018) The 2018 classification and checklist of lichenicolous fungi, with 2000 non-lichenized, obligately lichenicolous taxa. Bryologist 121:340–425. https://doi.org/10.1639/0007-2745-121.3.340
Dissanayake LS, Samarakoon MC, Mortimer PE, Lu YZ, Li QR, Hyde KD, Kang JC (2020) Morpho-molecular characterization of two novel amphisphaeriaceous species from Yunnan, China. Phytotaxa 446:144–158. https://doi.org/10.11646/phytotaxa.446.3.1
Dissanayake LS, Wijayawardene NN, Samarakoon MC, Hyde KD, Kang JC (2021) The taxonomy and phylogeny of Austropleospora ochracea sp. Nov. (Didymosphaeriaceae) from Guizhou, China. Phytotaxa 491(3):217–229. https://doi.org/10.11646/phytotaxa.491.3.2
Doilom M, Dissanayake AJ, Wanasinghe DN, Boonmee S, Liu JK, Bhat DJ, Taylor JE, Bahkali AH, McKenzie EHC, Hyde KD (2017) Microfungi on Tectona grandis (teak) in Northern Thailand. Fungal Divers 82:107–182. https://doi.org/10.1007/s13225-016-0368-7
Dong JY, Zhao ZX, Cai L, Liu SQ, Zhang HR, Duan M, Zhang KQ (2004) Nematicidal effect of freshwater fungal cultures against the pine-wood nematode, Bursaphelenchus xylophilus. Fungal Divers 15:123–133
Dong JY, Zhou YP, Li R, Zhou W, Li L, Zhu YH, Huang R, Zhang KQ (2006) Newnematicidal azaphilones fromtheaquatic fungus Pseudohalonectria adversaria YMF1.01019. FEMS Microbiol Lett 264:65–69. https://doi.org/10.1111/j.1574-6968.2006.00430.x
Dong W, Hyde KD, Bhat DJ, Zhang H (2018) Introducing Aculeata aquatica gen. et sp. nov., Minimelanolocus thailandensis sp. nov. and Thysanorea aquatica sp. nov. (Herpotrichiellaceae, Chaetothyriales) from freshwater in northern Thailand. Mycol Prog 17:617–629. https://doi.org/10.1007/s11557-018-1389-2
Dong W, Hyde KD, Doilom M, Yu XD, Bhat DJ, Jeewon R, Boonmee S, Wang GN, Nalumpang S, Zhang H (2020a) Pseudobactrodesmium (Dactylosporaceae, Eurotiomycetes, Fungi) a Novel Lignicolous Genus. Front Microbiol 11:456. https://doi.org/10.3389/fmicb.2020.00456
Dong W, Wang B, Hyde KD, McKenzie EHC, Raja HA, Tanaka K, Abdel-Wahab MA, Abdel-Aziz FA, Doilom M, Phookamsak R, Hongsanan S, Wanasinghe DN, Yu XD, Wang GN, Yang H, Yang J, Thambugala KM, Tian Q, Luo ZL, Yang JB, Miller AN, Fournier J, Boonmee S, Hu DM, Nalumpang S, Zhang H (2020b) Freshwater dothideomycetes. Fungal Divers 105:319–575. https://doi.org/10.1007/s13225-020-00463-5
Dong W, Hyde KD, Jeewon R, Doilom M, Yu XD, Wang GN, Liu NG, Hu DM, Nalumpang S, Zhang H (2021a) Towards a natural classification of annulatascaceae-like taxa II: introducing five new genera and eighteen new species from freshwater. Mycosphere 12(1):1–88. https://doi.org/10.5943/mycosphere/12/1/1
Dong W, Jeewon R, Hyde KD, Yang EF, Zhang H, Yu X, Wang G, Suwannarach N, Doilom M, Dong Z (2021b) Five novel taxa from freshwater habitats and new taxonomic insights of Pleurotheciales and Savoryellomycetidae. J Fungi 7:711. https://doi.org/10.3390/jof7090711
Dubey R, Manikpuri S (2021) Conlarium indicum: a novel fungus from Western Ghats of India. J Fungal Biol 11:112–118. https://doi.org/10.5943/cream/11/1/9
Dubey R, Pandey AD (2020) Mycoenterolobium borivaliense sp. Nov. (Pleosporomycetidae, Dothideomycetes) reported from India. J New Biol Rep 9(3):312–315
Ehrenberg (1819) Fungorum nova genera tria. Jahrbücher der Gewächskunde 1(2):51–58
El-Elimat T, Raja HA, Figueroa M, Al Sharie AH, Bunch RL, Oberlies NH (2021) Freshwater fungi as a source of chemical diversity: a review. J Nat Prod 84(3):898–916. https://doi.org/10.1021/acs.jnatprod.0c01340
Ellis MB (1958) Clasterosporium and some allied dematiaceae-phragmosporae I. Mycol Pap 70:1–89
Ellis MB (1960) Dematiaceous hyphomycetes. I. Mycol Pap 76:1–36
Ellis MB (1961) Dematiaceous hyphomycetes II. Mycol Pap 79:1–23
Ellis MB (1971) Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew
Ellis MB (1976) More dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, England
Eriksson OE (1983) Outline of the ascomycetes—1983. Systema Ascomycetum 2:1–37
Eriksson OE (2000) Notes on ascomycete systematics. Nos 2756–2939. Myconet 4:1–20
Eriksson OE, Hawksworth DL (1993) Outline of the ascomycetes. Systema Ascomycetum 12:51–257
Eriksson OE, Hawksworth DL (1998a) Outline of the ascomycetes. Systema Ascomycetum 16:83–296
Eriksson OE, Hawksworth DL (1998b) Notes on ascomycete systematics. Nos 2256–2439. Systema Ascomycetum 16:39–81
Fallah PM, Crane JL, Shearer CA (1999) Freshwater ascomycetes: two new species of Ascotaiwania from North America. Can J Bot 77(1):87–92. https://doi.org/10.1139/b98-202
Feeser I, O’Connell M (2010) Late Holocene land-use and vegetation dynamics in an upland karst region based on pollen and coprophilous fungal spore analyses: an example from the Burren, western Ireland. Veg Hist Archaeobot 19(5–6):409–426. https://doi.org/10.1007/s00334-009-0235-5
Feng JW, Liu WT, Chen JJ, Zhang CL (2021) Biogeography and ecology of Magnaporthales: a case study. Front Microbiol 12:654380. https://doi.org/10.3389/fmicb.2021.654380
Fernández FA, Huhndorf SM (2005) New species of Chaetosphaeria, Melanopsammella and Tainosphaeria gen. nov from the Americas. Fungal Divers 18:15–57
Fernández FA, Lutzoni FM, Huhndorf SM (1999) Teleomorph-anamorph connections: the new pyrenomycetous genus Carpoligna and its Pleurothecium anamorph. Mycologia 91:251–262. https://doi.org/10.1080/00275514.1999.12061015
Fernández FA, Miller AN, Huhndorf SM, Lutzoni FM, Zoller S (2006) Systematics of the genus Chaetosphaeria and its allied genera: morphological and phylogenetic diversity in north temperate and neotropical taxa. Mycologia 98:121–130. https://doi.org/10.1080/15572536.2006.11832718
Ferrer A, Miller AN, Shearer CA (2011) Minutisphaera and Natipusilla: two new genera of freshwater Dothideomycetes. Mycologia 103(2):411–423. https://doi.org/10.3852/10-177
Ferrer A, Miller AN, Sarmiento C, Shearer CA (2012) Three new genera representing novel lineages of Sordariomycetidae (Sordariomycetes, Ascomycota) from tropical freshwater habitats in Costa Rica. Mycologia 104:865–879. https://doi.org/10.3852/11-111
Foremska E, Kostecki M, Chelkowski J (1992) Biosynthesis, preparation and properties of ascochitine. Acta Biotechnol 12:461–465. https://doi.org/10.1002/abio.370120604
Fries EM (1849) Summa vegetabilium Scandinaviae II. Sectio posterior, Upsaliae
Fröhlich J, Hyde KD (2000) Palm microfungi. Fungal Divers Res Ser 3:1–393
Fuckel L (1870) Symbolae mycologicae. Beiträge zur Kenntniss der Rheinischen Pilze. Jahrbücher des Nassauischen Vereins für Naturkunde 23–24:1–459
Gams W, Holubová-Jechová V (1976) Chloridium and some other dematiaceous hyphomycetes growing on decaying wood. Stud Mycol 13:1–99
Gams W, Seifert KA, Morgan-Jones G (2009) New and validated hyphomycete taxa to resolve nomenclatural and taxonomic issues. Mycotaxon 110:89–108. https://doi.org/10.5248/110.89
García-García MA, Heredia G, García SC, Rosique-Gil E (2013) Analysis of the sporulating microfungal community in decomposing fallen leaves of Rinorea guatemalensis (Wats.) Bartlett (Malphigiales, Violaceae) in a Mexican Rainforest. Cryptogam Mycol 34(2):99–111. https://doi.org/10.7872/crym.v34.iss2.2013.99
Gharizadeh KH, Sheykholeslami A, Khodaparast SA (2007) A study of the identification of wood inhabiting hyphomycetes in Chalus vicinity (Iran). Rostaniha 8(1):30–32
Goh TK, Hyde KD (1998) A new hyphomycete genus, Conioscyphopsis, from wood submerged in a freshwater stream and a review of Conioscypha. Mycol Res 102:308–312. https://doi.org/10.1017/S0953756297004942
Goh TK, Hyde KD (1999) Fungi on submerged wood and bamboo in the Plover Cove Reservoir, Hong Kong. Fungal Divers 3:57–85
Goh TK, Ho WH, Hyde KD, Umali TM (1997a) New records and species of Sporoschisma and Sporoschismopsis from submerged wood in the tropics. Mycol Res 101:1295–1307. https://doi.org/10.1017/S0953756297003973
Goh TK, Ho WH, Hyde KD, Tsui KM (1997b) Four new species of Xylomyces from submerged wood. Mycol Res 101:1323–1328. https://doi.org/10.1017/S0953756297004164
Gönczöl J, Révay Á (2003) Treehole fungal communities: aquatic, aero-aquatic and dematiaceous hyphomycetes. Fungal Divers 12:19–34
Gönczöl J, Révay Á (2004) Fungal spores in rainwater: stemflow, throughfall and gutter conidial assemblages. Fungal Divers 16:67–86
Goos RD (1970) A new genus of the hyphomycetes from Hawaii. Mycologia 62:171–175. https://doi.org/10.1080/00275514.1970.12018951
Gorbushina AA, Whitehead K, Dornieden T, Niesse A, Schulte A, Hedges JI (2003) Black fungal colonies as units of survival: hyphal mycosporines synthesized by rock-dwelling microcolonial fungi. Can J Bot 81(2):131–138. https://doi.org/10.1139/b03-011
Grossart HP, Van den Wyngaert S, Kagami M, Wurzbacher C, Cunliffe M, Rojas-Jimenez K (2019) Fungi in aquatic ecosystems. Nat Rev Microbiol 17:339–354. https://doi.org/10.1038/s41579-019-0175-8
Guatimosim E, Firmino A, Bezerra J, Pereira O, Barreto R, Crous P (2015) Towards a phylogenetic reappraisal of Parmulariaceae and Asterinaceae (Dothideomycetes). Persoonia 35:230–241. https://doi.org/10.3767/003158515X688046
Gueidan C, Aptroot A, da Silva Cáceres ME, Badali H, Stenroos S (2014) A reappraisal of orders and families within the subclass Chaetothyriomycetidae (Eurotiomycetes, Ascomycota). Mycol Prog 13:1027–1039. https://doi.org/10.1007/s11557-014-0990-2
Halsted BD (1890) Some fungus disease of the sweet potato. New Jersey Agric Exp Stn Bull 76:25–27
Harter LL (1916) Sweet-potato scurf. J Agric Res 5:787–795
Hashimoto A, Matsumura M, Hirayama K, Tanaka K (2017) Revision of Lophiotremataceae (Pleosporales, Dothideomycetes): Aquasubmersaceae, Cryptocoryneaceae, and Hermatomycetaceae fam. nov. Persoonia 39:51–73. https://doi.org/10.3767/persoonia.2017.39.03
Hawksworth DL (1979) Ascospore sculpturing and generic concepts in the Testudinaceae (syn. Zopfiaceae). Can J Bot 57:91–99. https://doi.org/10.1139/b79-017
Hawksworth DL (1985) A redisposition of the species referred to the ascomycete genus Microthelia. Bull Br Mus Bot Ser 14:43–181
Hawksworth DL, Eriksson O (1986) The names of accepted orders of ascomycetes. Systema Ascomycetum 5:175–184
Hawksworth DL, Kirk PM, Sutton BC, Pegler DN (1995) Ainsworth & Bisby’s dictionary of the fungi, 8th edn. CABI International, Wallingford
Heredia G, Arias-Mota RM, Mena-Portales J, Castañeda-Ruiz RF (2018) Saprophytic synnematous microfungi. New records and known species for Mexico. Rev Mex Biodivers 89:604–618. https://doi.org/10.22201/ib.20078706e.2018.3.2352
Hernández-Gutiérrez A, Sutton BC (1997) Imimyces and Linkosia, two new genera segregated from Sporidesmium sensu lato, and redescription of Polydesmus. Mycol Res 101(2):201–209. https://doi.org/10.1017/S0953756296002419
Hernández-Restrepo M, Gené J, Castañeda-Ruiz RF, Mena-Portales J, Crous PW, Guarro J (2017) Phylogeny of saprobic microfungi from Southern Europe. Stud Mycol 86:53–97. https://doi.org/10.1016/j.simyco.2017.05.002
Hernández-Restrepo M, Giraldo A, van Doorn R, Wingfield MJ, Groenewald JZ, Barreto RW, Colmán AA, Mansur PSC, Crous PW (2020) The Genera of Fungi – G6: Arthrographis, Kramasamuha, Melnikomyces, Thysanorea, and Verruconis. Fungal Syst Evol 6:1–24. https://doi.org/10.3114/fuse.2020.06.01
Hirayama K, Tanaka K, Raja HA, Miller AN, Shearer CA (2010) A molecular phylogenetic assessment of Massarina ingoldiana sensu lato. Mycologia 102:729–746. https://doi.org/10.3852/09-230
Ho WH, Hyde KD (2004) A new type of conidial septal pore in fungi. Fungal Divers 15:71–186
Ho WH, Hyde KD, Hodgkiss IJ (1999a) Digitodesmium recurvum, a new species of chirosporous hyphomycete from Hong Kong. Mycologia 91:900–904. https://doi.org/10.1080/00275514.1999.12061096
Ho WH, Tsui CKM, Hodgkiss IJ, Hyde KD (1999b) Aquaticola, a new genus of Annulatascaceae from freshwater habitats. Fungal Divers 3:87–89
Ho WH, Hyde KD, Hodgkiss IJ (2004) Cataractispora receptaculorum, a new freshwater ascomycete from Hong Kong. Mycologia 96:411–417. https://doi.org/10.1080/15572536.2005.11832986
Holubová-Jechová V (1990) Problems in the taxonomy of the dematiaceous hyphomycetes. Stud Mycol 32:41–48
Holubová-Jechová V, Mercado-Sierra A (1984) Studies on Hyphomycetes from Cuba II. Hyphomycetes from the Isla de la Juventud. Ceská Mykol 38(2):96–120
Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Boonmee S, Lücking R, Pem D, Bhat DJ, Liu N, Tennakoon DS, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe I, Tibpromma S, Jayasiri SC, Sandamali D, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Dissanayake AJ, Luo ZL, Tian Q, Phukhamsakda C, Thambugala KM, Dai DQ, Chethana TKW, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Konta S, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-Gonzáles R, Aptroot A, Kashiwadani H, Harishchandra D, Aluthmuhandiram JVS, Abeywickrama PD, Bao DF, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Chomnunti P, Samarakoon MC, Suetrong S, Chaiwan N, Dayarathne MC, Jing Y, Rathnayaka AR, Bhunjun CS, Xu JC, Zheng JS, Liu G, Feng Y, Xie N (2020a) Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 11:1553–2107. https://doi.org/10.5943/mycosphere/11/1/13
Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Boonmee S, Lücking R, Pem D, Bhat DJ, Liu N, Tennakoon DS, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe I, Tibpromma S, Jayasiri SC, Sandamali D, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Phukhamsakda C, Thambugala KM, Dai DQ, Chethana TKW, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Konta S, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-Gonzáles R, Aptroot A, Kashiwadani H, Harishchandra D, Abeywickrama PD, Bao DF, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Chomnunti P, Samarakoon MC, Suetrong S, Chaiwan N, Dayarathne MC, Jing Y, Rathnayaka AR, Bhunjun CS, Xu JC, Zheng JS, Liu G, Feng Y, Xie N (2020b) Refned families of Dothideomycetes: Orders and families incertae sedis in Dothideomycetes. Fungal Divers 105:17–318. https://doi.org/10.1007/s13225-020-00462-6
Hongsanan S, Maharachchikumbura SSN, Hyde KD, Samarakoon MC, Jeewon R, Zhao Q, Al-Sadi AM, Bahkali AH (2017) An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers 84:25–41. https://doi.org/10.1007/s13225-017-0384-2
Hosagoudar V, Sabeena A, Mathew SP (2013) Additions to asterinaceous (Ascomycetes) fungi in India. J Threat Taxa 5:3670–3672. https://doi.org/10.11609/JoTT.o3228.3670-72
Hsieh SY, Goh TK, Kuo CH (2021) A taxonomic revision of Stanjehughesia (Chaetosphaeriaceae, Sordariomycetes), with a novel species S. kaohsiungensis from Taiwan. Phytotaxa 484(3):261–280. https://doi.org/10.11646/phytotaxa.484.3.2
Hu DM, Cai L, Bahkali AH, Hyde KD (2012) Two new freshwater species of Annulatascaceae from China. Mycotaxon 120:81–88. https://doi.org/10.5248/120.81
Hu DM, Liu F, Cai L (2013) Biodiversity of aquatic fungi in China. Mycology 4:125–168. https://doi.org/10.1080/21501203.2013.835752
Huang SK, Hyde KD, Mapook A, Maharachchikumbura SSN, Bhat DJ, McKenzie EHC, Jeewon R, Wen TC (2021) Taxonomic studies of some often over-looked Diaporthomycetidae and Sordariomycetidae. Fungal Divers 111:443–572. https://doi.org/10.1007/s13225-021-00488-4
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Hughes SJ (1949) Studies on micro-fungi. II. The genus Sporoschisma Berkeley & Broome and a redescription of Helminthosporium rousselianum Montagne. Mycol Pap 31:1–34
Hughes SJ (1951) Studies on micro-fungi. XII. Triposporium, Tripospermum, Ceratosporella and Tetraposporium (gen. nov.). Mycol Pap 46:1–35
Hughes SJ (1952) Fungi from the Gold Coast. I Mycol Pap 48:1–91
Hughes SJ (1966) New Zealand fungi. 6. Sporoschisma Berk. and Br. N Z J Bot 4:77–85
Hughes SJ (1978) New Zealand Fungi. 25 Miscellaneous species. N Z J Bot 16:311–370. https://doi.org/10.1080/0028825x.1978.10425143
Huhndorf SM, Fernández FA (2005) Teleomorph-anamorph connections: Chaetosphaeria raciborskii and related species, and their craspedodidymum-like anamorphs. Fungal Divers 19:23–49
Huhndorf SM, Miller AN, Fernandez FA (2004) Molecular systematics of the Coronophorales and new species of Bertia, Lasiobertia and Nitschkia. Mycol Res 108(12):1384–1398. https://doi.org/10.1017/S0953756204001273
Huhndorf SM, Greif M, Miller AN (2008) Two new genera in the Magnaporthaceae, a new addition to Ceratosphaeria and two new species of Lentomitella. Mycologia 100:940–955. https://doi.org/10.3852/08-037
Hyde KD (1991) Helicascus kanaloanus, Helicascus nypae sp. nov. and Salsuginea ramicola gen. et sp. nov. from intertidal mangrove wood. Bot Mar 34:311–318. https://doi.org/10.1515/botm.1991.34.4.311
Hyde KD (1992a) Tropical Australian freshwater fungi. II. Annulatascus velatispora gen. et sp. nov., A. bipolaris sp. nov. and Nais aquatica sp. nov. (Ascomycetes). Aust Syst Bot 5:117–124. https://doi.org/10.1071/SB9920117
Hyde KD (1992b) Fungi from decaying inter-tidal fronds of Nypa fruticans, including three new genera and four new species. Bot J Linn Soc 110:95–110. https://doi.org/10.1111/j.1095-8339.1992.tb00284.x
Hyde KD (1993) Tropical Australian freshwater fungi. VI*. Tiarosporella paludosa and Clohesyomyces aquaticus gen. et sp. nov. (Coelomycetes). Aust Syst Bot 6:169–173. https://doi.org/10.1071/SB9930169
Hyde KD, Goh TK (1998) Fungi on submerged wood in Lake Barrine, north Queensland, Australia. Mycol Res 102(6):739–749. https://doi.org/10.1017/s0953756297005868
Hyde KD, Jones EBG (1989) Ecological observations on marine fungi from the Seychelles. Bot J Linn Soc 100:237–254. https://doi.org/10.1111/j.1095-8339.1989.tb01720.x
Hyde KD, Jones EBG (1998) Marine mangrove fungi. Mar Ecol 9(1):15–33. https://doi.org/10.1111/j.1439-0485.1988.tb00196.x
Hyde KD, Goh TK, Steinke TD (1998) Fungi on submerged wood in the Palmiet River, Durban, South Africa. S Afr J Bot 64:151–162. https://doi.org/10.1016/S0254-6299(15)30860-7
Hyde KD, Wong SW, Jones EBG (1999) Cataractispora aquatica gen. et sp. nov. with three new freshwater lignicolous species. Mycol Res 103:1019–1031. https://doi.org/10.1017/s0953756298008065
Hyde KD, Ho WH, Jones EBG, Tsui CKM, Wong SW (2000) Torrentispora fibrosa gen. et sp. nov. (Annulatascaceae) from freshwater habitats. Mycol Res 104:399–1403. https://doi.org/10.1017/S0953756200002781
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, de Hoog GS, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M (2013) Families of dothideomycetes. Fungal Divers 63:1–313. https://doi.org/10.1007/s13225-013-0263-4
Hyde KD, Fryar S, Tian Q, Bahkali AH, Xu JC (2016a) Lignicolous freshwater fungi along a north–south latitudinal gradient in the Asian/Australian region; can we predict the impact of global warming on biodiversity and function? Fungal Ecol 19:190–200. https://doi.org/10.1016/j.funeco.2015.07.002
Hyde KD, Hongsanan S, Jeewon R, Bhat DJ, McKenzie EHC, Jones EBG, Phookamsak R, Ariyawansa HA, Boonmee S, Zhao Q, Abdel-Aziz FA, Abdel-Wahab MA, Banmai S, Chomnunti P, Cui BK, Daranagama DA, Das K, Dayarathne MC, de Silva NI, Dissanayake AJ, Doilom M, Ekanayaka AH, Gibertoni TB, GoesNeto A, Huang SK, Jayasiri SC, Jayawardena RS, Konta S, Lee HB, Li WJ, Lin CG, Liu JK, Lu YZ, Luo ZL, Manawasinghe IS, Manimohan P, Mapook A, Niskanen T, Norphanphoun C, Papizadeh M, Perera RH, Phukhamsakda C, Richter C, Santiago A, Drechsler-Santos ER, Senanayake IC, Tanaka K, Tennakoon T, Thambugala KM, Tian Q, Tibpromma S, Thongbai B, Vizzini A, Wanasinghe DN, Wijayawardene NN, Wu HX, Yang J, Zeng XY, Zhang H, Zhang JF, Bulgakov TS, Camporesi E, Bahkali AH, Amoozegar MA, Araujo-Neta LS, Ammirati JF, Baghela A, Bhatt RP, Bojantchev D, Buyck B, da Silva GA, de Lima CLF, de Oliveira RJV, de Souza CAF, Dai YC, Dima B, Duong TT, Ercole E, Mafalda-Freire F, Ghosh A, Hashimoto A, Kamolhan S, Kang JC, Karunarathna SC, Kirk PM, Kytovuori I, Lantieri A, Liimatainen K, Liu ZY, Liu XZ, Lucking R, Medardi G, Mortimer PE, Nguyen TTT, Promputtha I, Raj KNA, Reck MA, Lumyong S, Shahzadeh-Fazeli SA, Stadler M, Soudi MR, Su HY, Takahashi T, Tangthirasunun N, Uniyal P, Wang Y, Wen TC, Xu JC, Zhang ZK, Zhao YC, Zhou JL, Zhu L (2016b) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 80:1–270. https://doi.org/10.1007/s13225-016-0373-x
Hyde KD, Maharachchikumbura SSN, Hongsanan S, Samarakoon MC, Lücking R, Pem D, Harishchandra D, Jeewon R, Zhao RL, Xu JC (2017a) The ranking of fungi: a tribute to David L. Hawksworth on his 70th birthday. Fungal Divers 84:1–23. https://doi.org/10.1007/s13225-017-0383-3
Hyde KD, Norphanphoun C, Abreu VP, Bazzicalupo A, Chethana KWT, Clericuzio M, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, He MQ, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Karunarathna A, Konta S, Kusan I, Lee H, Li J, Lin CG, Liu NG, Lu YZ, Luo ZL, Manawasinghe IS, Mapook A, Perera RH, Phookamsak R, Phukhamsakda C, Siedlecki I, Soares AM, Tennakoon DS, Tian Q, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Abdel-Aziz FA, Li WJ, Senanayake IC, Shang QJ, Daranagama DA, de Silva NI, Thambugala KM, Abdel-Wahab MA, Bahkali AH, Berbee ML, Boonmee S, Bhat DJ, Bulgakov TS, Buyck B, Camporesi E, Castaneda-Ruiz RF, Chomnunti P, Doilom M, Dovana F, Gibertoni TB, Jadan M, Jeewon R, Jones EBG, Kang JC, Karunarathna SC, Lim YW, Liu JK, Liu ZY, Plautz HL Jr, Lumyong S, Maharachchikumbura SSN, Matocec N, McKenzie EHC, Mesic A, Miller D, Pawlowska J, Pereira OL, Promputtha I, Romero AI, Ryvarden L, Su HY, Suetrong S, Tkalcec Z, Vizzini A, Wen TC, Wisitrassameewong K, Wrzosek M, Xu JC, Zhao Q, Zhao RL, Mortimer PE (2017b) Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Divers 87:1–235. https://doi.org/10.1007/s13225-017-0391-3
Hyde KD, Chaiwan N, Norphanphoun C, Boonmee S, Camporesi E, Chethana KWT, Dayarathne MC, de Silva NI, Dissanayake AJ, Ekanayaka AH, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena R, Jiang HB, Karunarathna A, Lin CG, Liu JK, Liu NG, Lu YZ, Luo ZL, Maharachchimbura SSN, Manawasinghe IS, Pem D, Perera RH, Phukhamsakda C, Samarakoon MC, Senwanna C, Shang QJ, Tennakoon DS, Thambugala KM, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Zhang JF, Zhang SN, Bulgakov TS, Bhat DJ, Cheewangkoon R, Goh TK, Jones EBG, Kang JC, Jeewon R, Liu ZY, Lumyong S, Kuo CH, McKenzie EHC, Wen TC, Yan JY, Zhao Q (2018) Mycosphere notes 169–224. Mycosphere 9(2):271–430. https://doi.org/10.5943/mycosphere/9/2/8
Hyde KD, Tennakoon DS, Jeewon R, Bhat DJ, Maharachchikumbura SSN, Rossi W, Leonardi M, Lee HB, Mun HY, Houbraken J, Nguyen TTT, Jeon SJ, Frisvad JC, Wanasinghe DN, Lücking R, Aptroot A, Cáceres MES, Karunarathna SC, Hongsanan S, Phookamsak R, de Silva NI, Thambugala KM, Jayawardena RS, Senanayake IC, Boonmee S, Chen J, Luo ZL, Phukhamsakda C, Pereira OL, Abreu VP, Rosado AWC, Bart B, Randrianjohany E, Hofstetter V, Gibertoni TB, Soares AMdS, Plautz HL, Sotão HMP, Xavier WKS, Bezerra JDP, de Oliveira TGL, de Souza-Motta CM, Magalhães OMC, Bundhun D, Harishchandra D, Manawasinghe IS, Dong W, Zhang SN, Bao DF, Samarakoon MC, Pem D, Karunarathna A, Lin CG, Yang J, Perera RH, Kumar V, Huang SK, Dayarathne MC, Ekanayaka AH, Jayasiri SC, Xiao Y, Konta S, Niskanen T, Liimatainen K, Dai YC, Ji XH, Tian XM, Mešić A, Singh SK, Phutthacharoen K, Cai L, Sorvongxay T, Thiyagaraja V, Norphanphoun C, Chaiwan N, Lu YZ, Jiang HB, Zhang JF, Abeywickrama PD, Aluthmuhandiram JVS, Brahmanage RS, Zeng M, Chethana T, Wei D, Réblová M, Fournier J, Nekvindová J, do Barbosa Nascimento R, dos Santos JEF, de Oliveira NT, Li GJ, Ertz D, Shang QJ, Phillips AJL, Kuo CH, Camporesi E, Bulgakov TS, Lumyong S, Jones EBG, Chomnunti P, Gentekaki E, Bungartz F, Zeng XY, Fryar S, Tkalčec Z, Liang J, Li G, Wen TC, Singh PN, Gafforov Y, Promputtha I, Yasanthika E, Goonasekara ID, Zhao RL, Zhao Q, Kirk PM, Liu JK, Yan J, Mortimer PE, Xu J, Doilom M (2019) Fungal diversity notes 1036–1150: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 96:1–242. https://doi.org/10.1007/s13225-019-00429-2
Hyde KD, Norphanphoun C, Maharachchikumbura SSN, Bhat DJ, Jones EBG, Bundhun D, Chen YJ, Bao DF, Boonmee S, Calabon MS, Chaiwan N, Chethana KWT, Dai DQ, Dayarathne MC, Devadatha B, Dissanayake AJ, Dissanayake LS, Doilom M, Dong W, Fan XL, Goonasekara ID, Hongsanan S, Huang SK, Jayawardena RS, Jeewon R, Karunarathna A, Konta S, Kumar V, Lin CG, Liu JK, Liu NG, Luangsa-ard J, Lumyong S, Luo ZL, Marasinghe DS, McKenzie EHC, Niego AGT, Niranjan M, Perera RH, Phukhamsakda C, Rathnayaka AR, Samarakoon MC, Samarakoon SMBC, Sarma VV, Senanayake IC, Shang QJ, Stadler M, Tibpromma S, Wanasinghe DN, Wei DP, Wijayawardene NN, Xiao YP, Yang J, Zeng XY, Zhang SN, Xiang MM (2020a) Refined families of sordariomycetes. Mycosphere 11(1):305–1059. https://doi.org/10.5943/mycosphere/11/1/7
Hyde KD, Dong Y, Phookamsak R, Jeewon R, Bhat DJ, Jones EBG, Liu NG, Abeywickrama PD, Mapook A, Wei D, Perera RH, Manawasinghe IS, Pem D, Bundhun D, Karunarathna A, Ekanayaka AH, Bao DF, Li J, Samarakoon MC, Chaiwan N, Lin CG, Phutthacharoen K, Zhang SN, Senanayake IC, Goonasekara ID, Thambugala KM, Phukhamsakda C, Tennakoon DS, Jiang HB, Yang J, Zeng M, Huanraluek N, Liu JK, Wijesinghe SN, Tian Q, Tibpromma S, Brahmanage RS, Boonmee S, Huang SK, Thiyagaraja V, Lu YZ, Jayawardena RS, Dong W, Yang EF, Singh SK, Singh SM, Rana S, Lad SS, Anand G, Devadatha B, Niranjan M, Sarma VV, Liimatainen K, Aguirre-Hudson B, Niskanen T, Overall A, Alvarenga RLM, Gibertoni TB, Pfliegler WP, Horváth E, Imre A, Alves AL, da Silva Santos AC, Tiago PV, Bulgakov TS, Wanasinghe DN, Bahkali AH, Doilom M, Elgorban AM, Maharachchikumbura SSN, Rajeshkumar KC, Haelewaters D, Mortimer PE, Zhao Q, Lumyong S, Xu J, Sheng J (2020b) Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 100:5–277. https://doi.org/10.1007/s13225-020-00439-5
Hyde KD, Jeewon R, Chen YJ, Bhunjun CS, Calabon MS, Jiang HB, Lin CG, Norphanphoun C, Sysouphanthong P, Pem D, Tibpromma S, Zhang Q, Doilom M, Jayawardena RS, Liu JK, Maharachchikumbura SSN, Phukhamsakda C, Phookamsak R, Al-Sadi AM, Thongklang N, Wang Y, Gaforov Y, Jones EBG, Lumyong S (2020c) The numbers of fungi: is the descriptive curve lattening? Fungal Divers 103:219–271. https://doi.org/10.1007/s13225-020-00458-2
Hyde KD, Bao DF, Hongsanan S, Chethana KWT, Yang J, Suwannarach N (2021a) Evolution of freshwater Diaporthomycetidae (Sordariomycetes) provides evidence for five new orders and six new families. Fungal Divers 107:71–105. https://doi.org/10.1007/s13225-021-00469-7
Hyde KD, Suwannarach N, Jayawardena RS, Manawasinghe IS, Liao CF, Doilom M, Cai L, Zhao P, Buyck B, Phukhamsakda C, Su WX, Fu YP, Li Y, Zhao RL, He MQ, Li JX, Tibpromma S, Lu L, Tang X, Kang JC, Ren GC, Gui H, Hofstetter V, Ryoo R, Antonín V, Hurdeal VG, Gentikaki E, Zhang JY, Lu YZ, Senanayake IC, Yu FM, Zhao Q, Bao DF (2021b) Mycosphere notes 325–344—Novel species and records of fungal taxa from around the world. Mycosphere 12(1):1101–1156. https://doi.org/10.5943/mycosphere/12/1/14
Index Fungorum (2022) http://www.indexfungorum.org/names/names.asp
Ingold CT (1975) Conidia in the foam of two English streams. Trans Br Mycol Soc 65(3):522–527. https://doi.org/10.1016/s0007-1536(75)80058-1
Jaklitsch WM, Réblová M (2015) Savoryellaceae Jaklitsch & Réblová. Index Fungorum 209:1
Jaklitsch WM, Gardiennet A, Voglmayr H (2016) Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia 37:82–105. https://doi.org/10.3767/003158516x690475
Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat DJ, Buyck B, Cai L, Dai YC, Abd-Elsalam KA, Ertz D, Hidayat I, Jeewon R, Jones EBG, Bahkali AH, Karunarathna SC, Liu JK, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, McKenzie EHC, Moncalvo JM, Ghobad-Nejhad M, Nilsson H, Pang K, Pereira OL, Phillips AJL, Raspe O, Rollins AW, Romero AI, Etayo J, Selcuk F, Stephenson SL, Suetrong S, Taylor JE, Tsui CKM, Vizzini A, Abdel-Wahab MA, Wen TC, Boonmee S, Dai DQ, Daranagama DA, Dissanayake AJ, Ekanayaka AH, Fryar SC, Hongsanan S, Jayawardena RS, Li WJ, Perera RH, Phookamsak R, De Silva NI, Thambugala KM, Tian Q, Wijayawardene NN, Zhao RL, Zhao Q, Kang JC, Promputtha I (2015) The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers 74:3–18. https://doi.org/10.1007/s13225-015-0351-8
Jayasiri SC, Hyde KD, Jones EBG, Lu YZ (2017) Neohelicosporium fusisporum sp. nov. (Tubeufiaceae) and the first report of a sexual morph for the genus. Stud Fungi 2:210–217. https://doi.org/10.5943/sif/2/1/24
Jayasiri SC, Hyde KD, Jones EBG, McKenzie EHC, Jeewon R, Phillips AJL, Bhat DJ, Wanasinghe DN, Liu JK, Lu YZ, Kang JC, Xu JC, Karunarathna SC (2019) Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 10:1–186. https://doi.org/10.5943/mycosphere/10/1/1
Jeewon R, Hyde KD (2016) Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7:1669–1677. https://doi.org/10.5943/mycosphere/7/11/4
Jeewon R, Liew ECY, Hyde KD (2003) Molecular systematics of the Amphisphaeriaceae based on cladistic analyses of partial LSU rDNA gene sequences. Mycol Res 107:1392–1402. https://doi.org/10.1017/s095375620300875x
Jiang HB, Jeewon R, Karunarathna SC, Phukhamsakda C, Doilom M, Kakumyan P, Suwannarach N, Phookamsak R, Lumyong S (2021a) Reappraisal of Immotthia in Dictyosporiaceae, Pleosporales: introducing Immotthia bambusae sp. Nov. and Pseudocyclothyriella clematidis comb. et gen. nov. based on morphology and phylogeny. Front Microbiol 12:656235. https://doi.org/10.3389/fmicb.2021a.656235
Jiang HB, Hyde KD, Yang EF, Kakumyan P, Bahkali AH, Elgorban AM, Karunarathna SC, Phookamsak R, Lumyong S (2021b) Morphological and phylogenetic appraisal of Ophioceras (Ophioceraceae, Magnaportha). PLoS ONE 16(8):e0253853. https://doi.org/10.1371/journal.pone.0253853
Jie CY, Zhou QX, Zhao WS, Jiang YL, Hyde KD, McKenzie EHC, Wang Y (2013) A new Myrmecridium species from Guizhou, China. Mycotaxon 124:1–8. https://doi.org/10.5248/124.1
Johnson DA, Simmons EG, Miller JS, Stewart EL (2002) Taxonomy and pathology of Macrospora/Nimbya on some North American bulrushes (Scirpus spp.). Mycotaxon 84:413–428
Jones EBG, Eaton RA (1969) Savoryella lignicola gen. et sp. nov. from water-cooling towers. Trans Br Mycol Soc 52:161–174. https://doi.org/10.1016/s0007-1536(69)80169-5
Jones EBG, Hyde KD (1992) Taxonomic studies on Savoryella Jones et Eaton (Ascomycotina). Bot Mar 35:83–91. https://doi.org/10.1515/botm.1992.35.2.83
Jones EBG, Hyde KD, Pang KL (2014) Freshwater fungi and fungal-like organisms. Walter de Gruyter, Berlin
Jones EBG, To-anun C, Suetrong S, Boonyuen N (2016) Mycosphere Essays 12. Progress in the classification of the water-cooling tower ascomycete Savoryella and a tribute to John Savory: a review. Mycosphere 7(5):570–581. https://doi.org/10.5943/mycosphere/7/5/4
Kang JC, Kong RYC, Hyde KD (1998) Studies on the Amphisphaeriales 1. Amphisphaeriaceae (sensu stricto) and its phylogenetic relationships inferred from 5.8S rDNA and ITS2 sequences. Fungal Divers 1:147–157
Kang JC, Hyde KD, Kong RYC (1999) Studies on the Amphisphaeriales. The genera excluded from the Amphisphaeriaceae, Cainiaceae and Clypeosphaeriaceae. Fungal Divers 2:135–151. https://doi.org/10.1007/bf02464294
Karandikar KG, Singh PN, Singh SK (2015) Mycoenterolobium flabelliforme: a new anamorphic fungus from India. Plant Pathol Quar 5:49–51. https://doi.org/10.5943/ppq/5/2/3
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Katoh K, Rozewicki J, Yamada KD (2017) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. https://doi.org/10.3410/f.731078852.793536612
Kirk PM (1981) New or interesting microfungi II. Dematiaceous hyphomycetes from Esher Common, Surrey. Trans Br Mycol Soc 77:279–297. https://doi.org/10.1016/s0007-1536(81)80031-9
Kirk PM (1982) New or interesting microfungi IV. Dematiaceous hyphomycetes from Devon. Trans Br Mycol Soc 78:55–74. https://doi.org/10.1016/S0007-1536(81)80010-1
Kirk PM (1983) New or interesting microfungi IX. Dematiaceous hyphomycetes from Esher Common. Trans Br Mycol Soc 80:449–467. https://doi.org/10.1016/S0007-1536(83)80041-2
Kirk PM, Sutton BC (1985) A reassessment of the anamorph genus Chaetopsina (Hyphomycetes). Trans Br Mycol Soc 85:709–717. https://doi.org/10.1016/s0007-1536(85)80267-9
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Ainsworth & Bisby’s dictionary of the fungi, 9th edn. CAB International, Oxon
Kirschner R, Chen ZC, Oberwinkler F (2001) New records of ten species of hyphomycetes from Taiwan. Fungal Sci 16:47–62
Klaubauf S, Tharreau D, Fournier E, Groenewald JZ, Crous PW, de Vries RP, Lebrun MH (2014) Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Stud Mycol 79:85–120. https://doi.org/10.1016/j.simyco.2014.09.004
Knapp DG, Kovacs GM, Zajta E, Groenewald JZ, Crous PW (2015) Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 35:87–100. https://doi.org/10.3767/003158515x687669
Koch J (1982) Some lignicolous marine fungi from Sri Lanka. Nord J Bot 2(2):163–169. https://doi.org/10.1111/j.1756-1051.1982.tb01177.x
Kodsueb R, Jeewon R, Vijaykrishna D, Mckenzie EHC, Lumyong P, Lumyong S, Hyde KD (2006a) Systematic revision of Tubeufiaceae based on morphological and molecular data. Fungal Divers 21:105–130
Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EHC, Hyde KD, Jeewon R (2006b) The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia 98:571–583. https://doi.org/10.3852/mycologia.98.4.571
Kodsueb R, McKenzie EHC, Lumyong S, Hyde KD (2008) Fungal succession on woody litter of Magnolia liliifera (Magnoliaceae). Fungal Divers 30:55–72
Kohlmeyer J (1969) Marine fungi of Hawaii including the new genus Helicascus. Can J Bot 47:1469–1487. https://doi.org/10.1139/b69-210
Kohlmeyer J, Volkmann-Kohlmeyer B (1990) Revision of marine species of Didymosphaeria (Ascomycotina). Mycol Res 94:685–690. https://doi.org/10.1016/s0953-7562(09)80669-2
Kruys A, Huhndorf SM, Miller AN (2015) Coprophilous contributions to the phylogeny of Lasiosphaeriaceae and allied taxa within Sordariales (Ascomycota, Fungi). Fungal Divers 70:101–113. https://doi.org/10.1007/s13225-014-0296-3
Kuraku S, Zmasek CM, Nishimura O, Katoh K (2013) aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res 41:W22–W28. https://doi.org/10.1093/nar/gkt389
Kuthubutheen AJ, Nawawi A (1988) Two new species of Kionochaeta (Hyphomycetes) and K. ramifera from Malaysia. Trans Br Mycol Soc 90:437–444. https://doi.org/10.1016/s0007-1536(88)80153-0
Kuthubutheen AJ, Nawawi A (1991) A new species of Ceratosporella and Triposporium lambdaseptatum (Matsush.) comb. nov. from Malaysia. Mycol Res 95:158–162. https://doi.org/10.1016/s0953-7562(09)81005-8
Kutorga E, Iršėnaitė R, Iznova T, Kasparavičius J, Markovskaja S, Motiejūnaitė J (2013) Species diversity and composition of fungal communities in a Scots pine forest affected by the great cormorant colony. Acta Mycol 48(2):173–188. https://doi.org/10.5586/am.2013.019
Lad S, Rajeshkumar KC, Sruthi OP, Ashtekar N, Ansil PA, Verma RK, Wijayawardene NN (2022) Resolving the phylogenetic placement of Gangliostilbe in the family Xenospadicoidaceae (Xenospadicoidales). MycoAsia 2022/01
Lal SP (1987) A new species of Ophioceras Sacc. from India. Kavaka 15:7–8
Larget B, Simon DL (1999) Markov Chasin Monte Carlo algorithms for the bayesian analysis of phylogenetic trees. Mol Biol Evol 16:750–759. https://doi.org/10.1093/oxfordjournals.molbev.a026160
Larsson A (2014) AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30:3276–3278. https://doi.org/10.1093/bioinformatics/btu531
Lawrence DP, Rotondo F, Gannibal PB (2016) Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycol Prog 15:3. https://doi.org/10.1007/s11557-015-1144-x
Leal JA, Giménez-Abián MI, Canales Á, Jiménez-Barbero J, Bernabé M, Prieto A (2009) Cell wall polysaccharides isolated from the fungus Neotestudina rosatii, one of the etiologic agents of mycetoma in man. Glycoconj J 26(8):1047–1054. https://doi.org/10.1007/s10719-008-9224-7
Leuchtmann A (1984) Über Phaeosphaeria Miyake und andere bitunicate Ascomyceten mit mehrfach querseptierten Ascosporen. Sydowia 37:75–194
Li WJ, Bhat D, Camporesi E, Tian Q, Wijayawardene NN, Dai DQ, Phookamsak R, Chomnunti P, Bahkali AH, Hyde KD (2015) New asexual morph taxa in Phaeosphaeriaceae. Mycosphere 6:681–708. https://doi.org/10.5943/mycosphere/6/6/5
Li GJ, Hyde KD, Zhao RL, Hongsanan S, Abdel-Aziz FA, AbdelWahab MA, Alvarado P, Alves-Silva G, Ammirati JF, Ariyawansa HA, Baghela A, Bahkali AH, Beug M, Bhat DJ, Bojantchev D, Boonpratuang T, Bulgakov TS, Camporesi E, Boro MC, Ceska O, Chakraborty D, Chen JJ, Chethana KWT, Chomnunti P, Consiglio G, Cui BK, Dai DQ, Dai YC, Daranagama DA, Das K, Dayarathne MC, De Crop E, De Oliveira RJV, de Souza CAF, de Souza JI, Dentinger BTM, Dissanayake AJ, Doilom M, Drechsler-Santos ER, Ghobad-Nejhad M, Gilmore SP, Góes-Neto A, Gorczak M, Haitjema CH, Hapuarachchi KK, Hashimoto A, He MQ, Henske JK, Hirayama K, Iribarren MJ, Jayasiri SC, Jayawardena RS, Jeon SJ, Jerônimo GH, Jesus AL, Jones EBG, Kang JC, Karunarathna SC, Kirk PM, Konta S, Kuhnert E, Langer E, Lee HS, Lee HB, Li WJ, Li XH, Liimatainen K, Lima DX, Lin CG, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Lücking R, Lumbsch HT, Lumyong S, Leaño EM, Marano AV, Matsumura M, McKenzie EHC, Mongkolsamrit S, Mortimer PE, Nguyen TTT, Niskanen T, Norphanphoun C, O’Malley MA, Parnmen S, Pawłowska J, Perera RH, Phookamsak R, Phukhamsakda C, Pires-Zottarelli CLA, Raspé O, Reck MA, Rocha SCO, de Santiago ALCMA, Senanayake IC, Setti L, Shang QJ, Singh SK, Sir EB, Solomon KV, Song J, Srikitikulchai P, Stadler M, Suetrong S, Takahashi H, Takahashi T, Tanaka K, Tang LP, Thambugala KM, Thanakitpipattana D, Theodorou MK, Thongbai B, Thummarukcharoen T, Tian Q, Tibpromma S, Verbeken A, Vizzini A, Vlasák J, Voigt K, Wanasinghe DN, Wang Y, Weerakoon G, Wen HA, Wen TC, Wijayawardene NN, Wongkanoun S, Wrzosek M, Xiao YP, Xu JC, Yan JY, Yang J, Da Yang S, Hu Y, Zhang JF, Zhao J, Zhou LW, Peršoh D, Phillips AJL, Maharachchikumbura SSN (2016a) Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 78:1–237. https://doi.org/10.1007/s13225-016-0366-9
Li JF, Bhat DJ, Phookamsak R, Mapook A, Lumyong S, Hyde KD (2016b) Sporidesmioides thailandican gen. et sp. nov. (Dothideomycetes) from northern Thailand. Mycol Prog 15:1169–1178. https://doi.org/10.1007/s11557-016-1238-0
Li HH, Zhang K, Xia JW, Wang JY, Yang CL, Zhang XG (2017) Catenularia variegata sp. nov. from southern China, and a first Chinese record of Xylocladium clautriavii. Mycotaxon 132:621–634. https://doi.org/10.5248/132.621
Li J, Jeewon R, Mortimer PE, Doilom M, Phookamsak R, Promputtha I (2020) Multigene phylogeny and taxonomy of Dendryphion hydei and Torula hydei spp. Nov. from herbaceous litter in northern Thailand. PLoS ONE 15(2):e0228067. https://doi.org/10.1371/journal.pone.0228067
Li CX, Yu XD, Dong W, Hu DM, Boonmee S, Zhang H (2021a) Freshwater hyphomycetes in Sordariomycetes: two new species of Tainosphaeria (Chaetosphaeriaceae, Chaetosphaeriales) from Thailand. Phytotaxa 509(1):56–68. https://doi.org/10.11646/phytotaxa.509.1.2
Li WL, Bao DF, Liu NG, Hyde KD, Liu JK (2021b) Aquatisphaeria thailandica gen. et sp. Nov. (Tetraplosphaeriaceae, Pleosporales) from freshwater habitat in Thailand. Phytotaxa 513:118–128. https://doi.org/10.11646/phytotaxa.513.2.3
Lin CG, Mckenzie EHC, Bhat DJ, Liu JK, Hyde KD, Lumyong S (2018) Pseudodactylaria brevis sp. Nov. from Thailand confirms the status of Pseudodactylariaceae. Phytotaxa 369:241–250. https://doi.org/10.11646/phytotaxa.369.4.1
Lin CG, McKenzie EHC, Liu JK, Jones EBG, Hyde KD (2019) Hyaline-spored chaetosphaeriaceous hyphomycetes from Thailand and China, with a review of the family Chaetosphaeriaceae. Mycosphere 10(1):655–700. https://doi.org/10.5943/mycosphere/10/1/14
Link HF (1809) Observationes in Ordines plantarum naturales: dissertatio I.ma complectens Anandrarum ordines Epiphytas, Mucedines Gastromycos et Fungos. Der Gesellschaft Naturforschender Freunde zu Berlin 3:3–42
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808. https://doi.org/10.1093/oxfordjournals.molbev.a026092
Liu F, Hu DM, Cai L (2012) Canlarium duplumascospora gen. et. sp nov and Jobellisia guangdongensis sp. nov. from freshwater habitats in China. Mycologia 104:1178–1186. https://doi.org/10.3852/11-379
Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SSN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, Dsouza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe D, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis J, Li XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2015a) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197. https://doi.org/10.1007/s13225-015-0324-y
Liu XY, Udayanga D, Luo ZL, Chen LJ, Zhou DQ, Su HY, Hyde KD (2015b) Backbone tree for Chaetothyriales with four new species of Minimelanolocus from aquatic habitats. Fungal Biol 119:1046–1062. https://doi.org/10.1016/j.funbio.2015.08.005
Liu JK, Yang J, Maharachchikumbura SSN, McKenzie EHC, Jones EBG, Hyde KD, Liu ZY (2016) Novel chaetosphaeriaceous hyphomycetes from aquatic habitats. Mycol Prog 15:1157–1167. https://doi.org/10.1007/s11557-016-1237-1
Liu B, Zhang M, Bussmann WR, Liu H, Liu Y, Peng Y, Zu K, Zhao Y, Liu Z, Yu S (2018) Species richness and conservation gap analysis of karst areas: a case study of vascular plants from Guizhou, China. Glob Ecol Conserv 16:e00460. https://doi.org/10.1016/j.gecco.2018.e00460
Liu NG, Bhat DJ, Hyde KD, Liu JK (2019a) Conioscypha tenebrosa sp. nov. (Conioscyphaceae) from China and notes on Conioscypha species. Phytotaxa 413:159–171. https://doi.org/10.11646/phytotaxa.413.2.5
Liu NG, Hyde KD, Bhat DJ, Jumpathong J, Liu JK (2019b) Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China. Mycosphere 10(1):757–775. https://doi.org/10.5943/mycosphere/10/1/17
Liu LL, Liu NG, Yang J, Chen YY, Liu ZY (2020) Cucurbitinus gen. nov. (Halosphaeriaceae, Microascales), a new genus to accommodate Cucurbitinus constrictus comb. nov. and Cucurbitinus ibericus comb. nov. Phytotaxa 455(2):119–136. https://doi.org/10.11646/phytotaxa.455.2.4
Locquin M (1984) Mycologie Générale et Structurale. Masson, Paris
Lombard L, Van der Merwe NA, Groenewald JZ, Crous PW (2015) Generic concepts in Nectriaceae. Stud Mycol 80:189–245. https://doi.org/10.1016/j.simyco.2014.12.002
Lu YZ, Boonmee S, Liu JK, Hyde KD, McKenzie EH, Eungwanichayapant PD, Kang JC (2018a) Multi-gene phylogenetic analyses reveals Neohelicosporium gen. nov. and five new species of helicosporous hyphomycetes from aquatic habitats. Mycol Prog 17:631–646. https://doi.org/10.1007/s11557-017-1366-1
Lu YZ, Liu JK, Hyde KD, Jeewon R, Kang JC, Fan C, Boonmee S, Bhat DJ, Luo ZL, Lin CG, Eungwanichayapant PD (2018b) A taxonomic reassessment of Tubeufiales based on multi-locus phylogeny and morphology. Fungal Divers 92:131–344. https://doi.org/10.1007/s13225-018-0411-y
Lu YZ, Zhang JY, Lin CG, Luo ZL, Liu JK (2020) Pseudodactylaria fusiformis sp. Nov. from freshwater habitat in China. Phytotaxa 446:95–102. https://doi.org/10.11646/phytotaxa.446.2.2
Lumbsch HT, Huhndorf SM (2007) Outline of ascomycota–2007. Myconet 13:1–58
Lundqvist N (1972) Nordic Sordariaceae s. lat. Symb Botanicae Ups 20:1–374
Luo ZL, Maharachchikumbur SSN, Liu XY, Li SH et al (2015) Annulatascus saprophyticus sp. nov. and Pseudoannulatascus gen. nov. to accommodate Annulatascus biatriisporus (Annulatascales, Sordariomycetes) from Thailand. Phytotaxa 239:174–182. https://doi.org/10.11646/phytotaxa.239.2.6
Luo ZL, Yang J, Liu JK, Su HY, Bahkali AH, Hyde KD (2016a) Two new species of Helicascus (Morosphaeriaceae) from submerged wood in northern Thailand. Phytotaxa 270:182–190. https://doi.org/10.11646/phytotaxa.270.3.2
Luo ZL, Bao DF, Bhat DJ, Yang J, Chai HM, Li SH, Bahkali AH, Su HY, Hyde KD (2016b) Sporoschisma from submerged wood in Yunnan, China. Mycol Prog 15:1145–1155. https://doi.org/10.1007/s11557-016-1236-2
Luo ZL, Bhat DJ, Jeewon R, Boonmee S, Bao DF, Zhao YC, Chai HM, Su HY, Su XJ, Hyde KD (2017) Molecular phylogeny and morphological characterization of asexual fungi (Tubeufiaceae) from freshwater habitats in Yunnan, China. Cryptogam Mycol 38:27–53. https://doi.org/10.7872/crym/v38.iss1.2017.27
Luo ZL, Hyde KD, Bhat DJ, Jeewon R, Maharachchikumbura SSN, Bao DF, Li WL, Su XJ, Yang XY, Su HY (2018a) Morphological and molecular taxonomy of novel species Pleurotheciaceae from freshwater habitats in Yunnan, China. Mycol Prog 17:511–530. https://doi.org/10.1007/s11557-018-1377-6
Luo ZL, Hyde KD, Liu JK, Bhat DJ, Bao DF, Li WL, Su HY (2018b) Lignicolous freshwater fungi from China II: novel Distoseptispora (Distoseptisporaceae) species from northwestern Yunnan Province and a suggested unified method for studying lignicolous freshwater fungi. Mycosphere 9:444–461. https://doi.org/10.5943/mycosphere/9/3/2
Luo ZL, Hyde KD, Liu JK, Maharachchikumbura SSN, Jeewon R, Bao DF, Bhat DJ, Lin CG, Li WL, Yang J, Liu NG, Lu YZ, Jayawardena RS, Li JF, Su HY (2019) Freshwater sordariomycetes. Fungal Divers 99:451–660. https://doi.org/10.1007/s13225-019-00438-1
Ma J (2016) Corynesporopsis obclavata and Stanjehughesia jiangxiensis spp. Nov. from Lushan Mountain, China. Mycotaxon 131(3):583–588. https://doi.org/10.5248/131.583
Ma LG, Xia JW, Ma YR, Castañeda-Ruíz RF, Zhang XG (2014) Two new species of Spadicoides and Gangliostilbe from southern China. Mycol Prog 13:547–552. https://doi.org/10.1007/s11557-013-0937-z
Magaña-Dueñas V, Stchigel AM, Cano-Lira JF (2020) New taxa of the family Amniculicolaceae (Pleosporales, Dothideomycetes, Ascomycota) from freshwater habitats in Spain. Microorganisms 8:1–15. https://doi.org/10.3390/microorganisms8091355
Magaña-Dueñas V, Cano-Lira JF, Stchigel AM (2022) Novel freshwater ascomycetes from Spain. J Fungi 8(8):849. https://doi.org/10.3390/jof8080849
Magyar D, Révay Á (2009) New species of Oncopodiella (Hyphomycetes) from living trees. Nova Hedwig 88(1–2):169–182. https://doi.org/10.1127/0029-5035/2009/0088-0169
Magyar D, Gönczöl J, Révay Á, Grillenzoni F, Seijo-Coello MDC (2005) Stauroand scolecoconidia in floral and honeydew honeys. Fungal Divers 20:103–120
Magyar D, Frenguelli G, Bricchi E, Tedeschini E, Csontos P, Li DW, Bobvos J (2009) The biodiversity of air spora in an Italian vineyard. Aerobiologia 25:99–109. https://doi.org/10.1007/s10453-009-9115-9
Magyar D, Shoemaker RA, Bobvos J, Crous PW, Groenewald JZ (2011a) Pyrigemmula, anovel hyphomycete genus on grapevine and tree bark. Mycol Prog 10:307–314. https://doi.org/10.1007/s11557-010-0703-4
Magyar D, Eszéki ER, Gy O, Szécsi Á, Kredics L, Hatvani L, Körmöczi P (2011b) The air spora of an orchid greenhouse. Aerobiologia 27(2):121–134. https://doi.org/10.1007/s10453-010-9182-y
Magyar D, Mura-Mészáros A, Grillenzoni F (2016) Fungal diversity in floral and honeydew honeys. Acta Bot Hung 58:145–166. https://doi.org/10.1556/034.58.2016.1-2.6
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang SK, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu JK, Liu ZY, Norphanphoun C, Pang KL, Perera RH, Senanayake IC, Shang Q, Shenoy BD, Xiao Y, Bahkali AH, Kang J, Somrothipol S, Suetrong S, Wen T, Xu J (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72:199–301. https://doi.org/10.1007/s13225-015-0331-z
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat JD, Dayarathne MC, Huang SK, Norphanphoun C, Senanayake IC, Perera RH, Shang QJ, Xiao YP, D’souza MJ, Hongsanan S, Jayawardena RS, Daranagama DA, Konta S, Goonasekara ID, Zhuang WY, Jeewon R, Phillips AJL, Abdel-Wahab MA, Al-Sadi AM, Bahkali AH, Boonmee S, Boonyuen N, Cheewangkoon R, Dissanayake AJ, Kang J, Li QR, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Pang KL, Phookamsak R, Promputtha I, Suetrong S, Stadler M, Wen TC, Wijayawardene NN (2016) Families of sordariomycetes. Fungal Divers 79:1–317. https://doi.org/10.1007/s13225-016-0369-6
Maharachchikumbura SSN, Luo ZL, Su HY, Al-Sadi AM, Cheewangkoon R (2018) Reticulascaceae hyphomycetes from submerged wood in Yunnan, China. Phytotaxa 348:187–198. https://doi.org/10.11646/phytotaxa.348.3.2
Manawasinghe IS, Calabon MS, Jones EBG, Zhang YX, Liao CF, Xiong Y, Chaiwan N, Kularathnage ND, Liu NG, Tang SM, Sysouphanthong P, Du TY, Luo M, Pasouvang P, Pem D, Phonemany M, Ishaq M, Chen JW, Karunarathna SC, Mai ZL, Rathnayaka AR, Samarakoon MC, Tennakoon DS, Wijesinghe SN, Yang YH, Zhao HJ, Fiaz M, Doilom M, Dutta AK, Khalid AN, Liu JW, Thongklang N, Senanayake IC, Tibpromma S, You LQ, Camporesi E, Gafforov YS, Hyde KD (2022) Mycosphere notes 345–386. Mycosphere 13(1):454–557. https://doi.org/10.5943/mycosphere/13/1/3
Manoharachary C, Rao NK, Agarwal DK, Kunwar IK (2006) Two new species of Acrodictys M.B. Ellis from India. Indian Phytopathol 59(1):91–93
Manoharachary C, Kunwar IK, Kumar GS, Reddy BS (2010) Two new species of Actinocladium Ehrenb. from India. Indian Phytopathol 63:83–84
Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ (2008) Microfungi occurring on the Proteaceae in the fynbos. CBS Fungal Biodiversity Centre, Utrecht
Marques MFO, Moraes-Júnior VO, Santos SML (2007) Fungos conidiais lignícolas em um fragmento de Mata Atlântica, Serra da Jibóia, BA. Revista Brasileira de Biociências 5(2):1186–1188
Matsushima T (1975) Icones fungorum a Matushima lectorum. Privately printed, Kobe
Matsushima T (1980) Matsushima Mycological Memoirs 1. Matsushima Fungus Collection, Kobe
Matsushima T (1983) Matsushima Mycological Memoirs 3. Matsushima Fungus Collection. Kobe. https://doi.org/10.2307/3793127
Matsushima T (1993) Matsushima Mycological Memoirs 7. Matsushima Fungus Collection, Kobe
Matsushima T (2003) [2001] Matsushima mycological memoirs 10. Matsushima Fungus Collection, Kobe
McKenzie EHC (1995) Dematiaceous Hyphomycetes on Freycinetia (Pandanaceae). 5. Sporidesmium sensu lato. Mycotaxon 56:9–29
Mehrabi M, Hemmati R, Asgari B (2017) Kirschsteiniothelia arasbaranica sp. nov., and an Emendation of the Kirschsteiniotheliaceae. Cryptogam Mycol 38(1):13–25. https://doi.org/10.7872/crym/v38.iss1.2017.13
Meyers SP, Moore RT (1960) Thalassiomycetes II. New genera and species of Deuteromycetes. Am J Bot 47:345–349. https://doi.org/10.1002/j.1537-2197.1960.tb07134.x
Miller AN, Huhndorf SM (2004) A natural classification of Lasiosphaeria based on nuclear LSU rDNA sequences. Mycol Res 108:26–34. https://doi.org/10.1017/s0953756203008864
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, pp 1–8. https://doi.org/10.1109/gce.2010.5676129
Minoura K, Muroi T (1978) Some freshwater ascomycetes from Japan. Trans Mycol Soc Jpn 19:129–134
Morin L, Shivas RG, Piper MC, Tan YP (2010) Austropleospora osteospermi gen. et sp. nov. and its host specificity and distribution on Chrysanthemoides monilifera ssp. rotundata in Australia. Fungal Divers 40:65–74. https://doi.org/10.1007/s13225-009-0007-7
Moustafa AF, Abdul-Wahid OA (1990) Veronaea constricta, a new Hyphomycete from Egyptian soils. Mycotaxon 38:167–171
Mugambi GK, Huhndorf SM (2008) A new species of Melanochaeta from Kenya. Sydowia 60:261–266
Müller E, Samuels GJ (1982a) Anamorphs of pyrenomycetous Ascomycetes I. Rhamphoria Niessl and Trichosphaerella Bommer, Rousseau & Saccardo. Sydowia 35:143–149
Müller E, Samuels GJ (1982b) Anamorphs of pyrenomycetous Ascomycetes. II. Porosphaerella gen. nov. and its Cordana anamorph. Sydowia 35:150–154
Müller E, Samuels GJ (1982c) Anamorphs of pyrenomycetous ascomycetes. III. The Sporoschisma and Chalara anamorphs of Melanochaeta aotearoae. Sydowia 35:155–161
Munk A (1953) The system of the pyrenomycetes. A contribution to a natural classification of the group Sphaeriales sensu Lindau. Dansk Botanisk Arkiv 15:1–163
Mustafa K, Abdullah SK (2011) The coprophilous genera Arnium and Cercophora from North Iraq. 2nd Scientific Conference for Biological Science, Mosul University
Nag Raj TR (1993) Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo. https://doi.org/10.2307/3760664
Nakagiri A (1993) Intertidal mangrove fungi from Iriomote Island. IFO Res Commun 16:24–62
Niessl G (1876) Notizen üeber neue und kritische Pyrenomyceten. Verh Naturforsch Ver Brünn 14:165–218
Nóbrega TF, Ferreira BW, Barreto RW (2021) Digitodesmium polybrachiatum sp. Nov., a new species of Dictyosporiaceae from Brazil. Mycol Prog 20:1135–1144. https://doi.org/10.21203/rs.3.rs-433487/v1
Nylander J (2008) MrModeltest2 v. 2.3 (Program for Selecting DNA Substitution Models Using PAUP*). Evolutionary Biology Centre, Uppsala
Ono Y, Kobayashi T (2001) Notes on new and noteworthy plant-inhabiting fungi from Japan (1). Mycoscience 42:439–446. https://doi.org/10.1007/bf02464340
Park JH, Choi GJ, Jang KS, Lim HK, Kim HT, Cho KY, Kim JC (2005) Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol Lett 252:309–313. https://doi.org/10.1016/j.femsle.2005.09.013
Pawłowska J, Łukasz I, Gorczak M, Galera H, Wrzosek M, Hawksworth DL (2017) Psychronectria hyperantarctica, gen. nov., comb. nov., epitypification and phylogenetic position of an Antarctic bryophilous ascomycete. Mycologia 109:601–607. https://doi.org/10.1080/00275514.2017.1398575
Pedro EG, Bononi VLR (2007) Cave fungi of the karst region of the State Touristic Park of the Upper Ribeira Valley (Petar) in the State of Sao Paulo in Brazil. Focus 5:65–78
Peintner U, Knapp M, Fleischer V, Walch G, Dresch P (2016) Myrmecridium hiemale sp. nov. from snow-covered alpine soil is the first eurypsychrophile in this genus of anamorphic fungi. Int J Syst Evol Microbiol 66:2592–2598. https://doi.org/10.1099/ijsem.0.001090
Pem D, Jeewon R, Bhat DJ, Doilom M, Boonmee S, Hongsanan S, Promputtha I, Xu JC, Hyde KD (2019) Mycosphere notes 275–324: a morpho-taxonomic revision and typification of obscure Dothideomycetes genera (incertae sedis). Mycosphere 10(1):1115–1246. https://doi.org/10.5943/mycosphere/10/1/22
Perera RH, Maharachchikumbura SSN, Ariyawansa H, Bahkali AH, Jones EBG, Al-Sadi AM, Hyde KD, Liu ZY (2016a) Two new Pseudohalonectria species on beech cupules (Fagus sylvatica) and a new genus to accommodate P. suthepensis. Phytotaxa 278:115–131. https://doi.org/10.11646/phytotaxa.278.2.2
Perera RH, Maharachchikumbura SSN, Bhat JD, Al-Sadi AM, Liu JK, Hyde KD, Liu ZY (2016b) New species of Thozetella and Chaetosphaeria and new records of Chaetosphaeria and Tainosphaeria from Thailand. Mycosphere 7:1301–1321. https://doi.org/10.5943/mycosphere/7/9/5
Petrak F (1923) Mykologische Notizen VI. Ann Mycol 21:182–335
Phookamsak R, Li WJ, Dai DQ, Singtripop C, Hyde KD (2015a) Poaceascoma helicoides gen et sp. nov., a new genus with scolecospores in Lentitheciaceae. Cryptogam Mycol 36:225–236. https://doi.org/10.7872/crym/v36.iss2.2015.225
Phookamsak R, Norphanphoun C, Tanaka K, Dai DQ, Luo ZL, Liu JK, Su HY, Bhat DJ, Bahkali AH, Mortimer PE (2015b) Towards a natural classification of Astrosphaeriella-like species; introducing Astrosphaeriellaceae and Pseudoastrosphaeriellaceae fam. nov. and Astrosphaeriellopsis, gen. nov. Fungal Divers 74:143–197. https://doi.org/10.1007/s13225-015-0352-7
Phookamsak R, Hyde KD, Jeewon R, Bhat DJ, Jones EBG, Maharachchikumbura SSN, Raspé O, Karunarathna SC, Wanasinghe DN, Hongsanan S, Doilom M, Tennakoon DS, Machado AR, Firmino AL, Ghosh A, Karunarathna A, Mešić A, Dutta AK, Thongbai B, Devadatha B, Norphanphoun C, Senwanna C, Wei D, Pem D, Ackah FK, Wang GN, Jiang HB, Madrid H, Lee HB, Goonasekara ID, Manawasinghe IS, Kušan I, Cano J, Gené J, Li J, Das K, Acharya K, Raj KNA, Latha KPD, Chethana KWT, He MQ, Dueñas M, Jadan M, Martín MP, Samarakoon MC, Dayarathne MC, Raza M, Park MS, Telleria MT, Chaiwan N, Matočec N, de Silva NI, Pereira OL, Singh PN, Manimohan P, Uniyal P, Shang QJ, Bhatt RP, Perera RH, Alvarenga RLM, Nogal-Prata S, Singh SK, Vadthanarat S, Oh SY, Huang SK, Rana S, Konta S, Paloi S, Jayasiri SC, Jeon SJ, Mehmood T, Gibertoni TB, Nguyen TTT, Singh U, Thiyagaraja V, Sarma VV, Dong W, Yu XD, Lu YZ, Lim YW, Chen Y, Tkalčec Z, Zhang ZF, Luo ZL, Daranagama DA, Thambugala KM, Tibpromma S, Camporesi E, Bulgakov TS, Dissanayake AJ, Senanayake IC, Dai DQ, Tang LZ, Khan S, Zhang H, Promputtha I, Cai L, Chomnunti P, Zhao RL, Lumyong S, Boonmee S, Wen TC, Mortimer PE, Xu JC (2019) Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers 95:1–273. https://doi.org/10.1007/s13225-019-00421-w
Phosri C, Põlme S, Taylor AFS, Kõljalg U, Suwannasai N, Tedersoo L (2012) Diversity and community composition of ectomycorrhizal fungi in a dry deciduous dipterocarp forest in Thailand. Biodivers Conserv 21:2287–2298. https://doi.org/10.1007/s10531-012-0250-1
Phukhamsakda C, Hongsanan S, Ryberg M, Ariyawansa HA, Chomnunti P, Bahkali AH, Hyde KD (2016) The evolution of Massarineae with Longipedicellataceae fam. nov. Mycosphere 7:1713–1731. https://doi.org/10.5943/mycosphere/7/11/7
Phukhamsakda C, McKenzie EHC, Phillips AJL, Jones EBG, Bhat DJ, Stadler M, Bhunjun CS, Wanasinghe DN, Thongbai B, Camporesi E, Ertz D, Jayawardena RS, Perera RH, Ekanayake AH, Tibpromma S, Doilom M, Xu JC, Hyde KD (2020) Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers 102:1–203. https://doi.org/10.1007/s13225-020-00448-4
Phukhamsakda C, Nilsson RH, Bhunjun CS, Gomes de Farias AR, Sun YR, Wijesinghe SN, Raza M, Bao DF, Lu L, Tibpromma S, Dong W, Tennakoon DS, Tian XG, Xiong YR, Karunarathna SC, Cai L, Luo ZL, Wang Y, Manawasinghe IS, Camporesi E, Kirk PM, Promputtha I, Kuo CH, Su HY, Doilom M, Li Y, Fu YP, Hyde KD (2022) The numbers of fungi: contributions from traditional taxonomic studies and challenges of metabarcoding. Fungal Divers 114:327–386. https://doi.org/10.1007/s13225-022-00502-3
Pinnoi A, Phongpaichit S, Hyde KD, Jones EBG (2009) Biodiversity of fungi on Calamus (Palmae) in Thailand. Cryptogam Mycol 30:181–190
Pino-Bodas R, Zhurbenko MP, Stenroos S (2017) Phylogenetic placement within Lecanoromycetes of lichenicolous fungi associated with Cladonia and some other genera. Persoonia 39:91–117. https://doi.org/10.3767/persoonia.2017.39.05
Pirozynski KA (1963) Beltrania and related genera. Mycol Pap 90:1–37
Pratibha J, Prabhugaonkar A (2015) Multi-gene phylogeny of Pithomyces with the sexual morph of P. flavus Berk. & Broome. Phytotaxa 218:84–90. https://doi.org/10.11646/phytotaxa.218.1.7
Preedanon S, Klaysuban A, Suetrong S, Promchoo W, Gundool W, Sangtiean T, Sakayaroj J (2017) Helicascus mangrovei sp. nov., a new intertidal mangrove fungus from Thailand. Mycoscience 58:174–180. https://doi.org/10.1016/j.myc.2017.01.001
Promputtha I, Lumyong S, Lumyong P, McKenzie EHC, Hyde KD (2004) A new species of Pseudohalonectria from Thailand. Cryptogam Mycol 25:43–47
Qin K, Zhu L, Wang J (2010) Screening and degradation characteristics of cypermethrin degrading fungi. Chin J Environ Eng 4(4):950–954
Quaedvlieg W, Verkley GJM, Shin HD, Barreto RW, Alfenas AC, Swart WJ, Groenewald JZ, Crous PW (2013) Sizing up Septoria. Stud Mycol 75:307–390. https://doi.org/10.3114/sim0017
Raja HA, Miller AN, Shearer CA (2012) Freshwater ascomycetes: Natipusillaceae, a new family of tropical fungi, including Natipusilla bellaspora sp. nov. from the Peruvian Amazon. Mycologia 104:569–573. https://doi.org/10.3852/11-150
Rambelli A, Onofri S (1987) New species of Kylindria and Xenokylindria and notes on Cylindrotrichum (Hyphomycetes). Trans Br Mycol Soc 88:393–397. https://doi.org/10.1016/s0007-1536(87)80012-8
Rambelli A, Tempesta S, Venturella G, Ciccarone C (2010) Dematiaceous Hyphomycetes from Pantelleria Mediterranean maquis litter. Third Contribution. Flora Mediterr 20:211–233
Ranghoo VM (1998) Phylogeny of freshwater ascomycetes. Dissertation, University of Hong Kong
Ranghoo VM, Hyde KD (1998) Ascolacicola aquatica gen. et sp. nov. and a new species of Ascotaiwania from wood submerged in a reservoir in Hong Kong. Mycologia 90:1055–1062. https://doi.org/10.2307/3761280
Ranghoo VM, Hyde KD, Liew ECY, Spatafora JW (1999) Family placement of Ascotaiwania and Ascolacicola based on DNA sequences from the large subunit rRNA gene. Fungal Divers 2:159–168
Ranghoo VM, Tsui CKM, Hyde KD (2001) Brunneosporella aquatica gen. et sp. nov., Aqualignicola hyalina gen. et sp. nov., Jobellisia viridifusca sp. nov. and Porosphaerellopsis bipolaris sp. nov. (ascomycetes) from submerged wood in freshwater habitats. Mycol Res 105:625–633. https://doi.org/10.1017/s0953756201003793
Rao D, Rao PR (1964) Sporoschisma Berk. & Br. from India. Mycopathol Mycol Appl 24:81–84. https://doi.org/10.1007/BF02075548
Rao VG, de Hoog GS (1986) New or critical Hyphomycetes from India. Stud Mycol 28:1–84
Rao VG, Reddy KA (1981) Two new hyphomycetes. Indian J Bot 4(1):108–114
Réblová M (1999) Studies in Chaetosphaeria sensu lato III. Umbrinosphaeria gen. nov. and Miyoshiella with Sporidesmium anamorphs. Mycotaxon 71:13–43
Réblová M (2000) The genus Chaetosphaeria and its anamorphs. Stud Mycol 45:149–168
Réblová M (2006) Molecular systematics of Ceratostomella sensu lato and morphologically similar fungi. Mycologia 98(1):68–93. https://doi.org/10.1080/15572536.2006.11832714
Réblová M (2009) Teleomorph of Rhodoveronaea (Sordariomycetidae) discovered and reevaluation of Pleurophragmium. Fungal Divers 36:129–139
Réblová M (2014) Sporoschismopsis angustata sp. nov., a new holomorph species in the Reticulascaceae (Glomerellales), and a reappraisal of Sporoschismopsis. Mycol Prog 13:671–681. https://doi.org/10.1007/s11557-013-0949-8
Réblová M, Gams W (1999) Teleomorph-anamorph connections in Ascomycetes 1. Cylindrotrichum and Cacumisporium anamorphs of Chaetosphaeria. Czech Mycol 51:1–40. https://doi.org/10.33585/cmy.51101
Réblová M, Gams W (2000) Life-history of Ascomycetes: two new species of Chaetosphaeria with Chloridium and Chloridium-Dictyochaeta anamorphs. Mycoscience 42:129–138. https://doi.org/10.1007/bf02464321
Réblová M, Seifert KA (2004) Conioscyphascus, a new ascomycetous genus for holomorphs with Conioscypha anamorphs. Stud Mycol 50:95–108
Réblová M, Seifert KA (2011) Discovery of the teleomorph of the hyphomycete, Sterigmatobotrys macrocarpa, and epitypification of the genus to holomorphic status. Stud Mycol 68:193–202. https://doi.org/10.3114/sim.2011.68.08
Réblová M, Štěpánek V (2018) Introducing the Rhamphoriaceae, fam. nov. (Sordariomycetes), two new genera, and new life histories for taxa with Phaeoisaria- and Idriella-like anamorphs. Mycologia 110:750–770. https://doi.org/10.1080/00275514.2018.1475164
Réblová M, Winka K (2000) Phylogeny of Chaetosphaeria and its anamorphs based on morphological and molecular data. Mycologia 92:939–954. https://doi.org/10.2307/3761589
Réblová M, Winka K (2001) Generic concepts and correlations in ascomycetes based on molecular and morphological data: Lecythothecium duriligni gen. et sp. nov. with a Sporidesmium anamorph, and Ascolacicola austriaca sp. nov. Mycologia 93:478–493. https://doi.org/10.2307/3761734
Réblová M, Barr ME, Samuels GJ (1999) Chaetosphaeriaceae, a new family for Chaetosphaeria and its relatives. Sydowia 51:49–70
Réblová M, Gams W, Seifert KA (2011a) Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Stud Mycol 68:163–191. https://doi.org/10.3114/sim.2011.68.07
Réblová M, Gams W, Štěpánek V (2011b) The new hyphomycete genera Brachyalara, and Infundichalara, the similar Exochalara, and species of ‘Phialophora, sect. Catenulatae’ (Leotiomycetes). Fungal Divers 46:67–86. https://doi.org/10.1007/s13225-010-0077-6
Réblová M, Seifert KA, Fournier J, Štěpánek V (2012) Phylogenetic classification of Pleurothecium and Pleurotheciella gen. nov. and its dactylaria-like anamorph (Sordariomycetes) based on nuclear ribosomal and protein-coding genes. Mycologia 104:1299–1314. https://doi.org/10.3852/12-035
Réblová M, Untereiner WA, Réblová K (2013) Novel evolutionary lineages revealed in the Chaetothyriales (fungi) based on multigene phylogenetic analyses and comparison of ITS secondary structure. PLoS ONE 8(5):e63547. https://doi.org/10.1371/journal.pone.0063547
Réblová M, Fournier J, Štěpánek V (2016a) Two new lineages of aquatic ascomycetes: Atractospora gen. nov. and Rubellisphaeria gen. et sp. nov., and a sexual morph of Myrmecridium montsegurinum sp. nov. Mycol Prog 15:21. https://doi.org/10.1007/s11557-016-1166-z
Réblová M, Miller AN, Rossman AY, Seifert KA, Crous PW, Hawksworth DL, Abdel-Wahab MA, Cannon PF, Daranagama DA, De Beer ZW, Huang SK, Hyde KD, Jayawardena RS, Jaklitsch W, Jones EBG, Ju YM, Judith C, Maharachchikumbura SSN, Pang KL, Petrini LE, Raja HA, Romero AI, Shearer C, Senanayake IC, Voglmayr H, Weir BS, Wijayawardene NN (2016b) Recommendations for competing sexual-asexually typified generic names in Sordariomycetes (except Diaporthales, Hypocreales, and Magnaporthales). IMA Fungus 7(1):131–153. https://doi.org/10.5598/imafungus.2016.07.01.08
Réblová M, Seifert KA, Fournier J, Štěpánek V (2016c) Newly recognized lineages of perithecial ascomycetes: the new orders Conioscyphales and Pleurotheciales. Persoonia 37:57–81. https://doi.org/10.3767/003158516x689819
Réblová M, Untereiner WA, Štìpánek V, Gams W (2017) Disentangling Phialophora section Catenulatae: disposition of taxa with pigmented conidiophores and recognition of a new subclass, Sclerococcomycetidae (Eurotiomycetes). Mycol Prog 16:27–46. https://doi.org/10.1007/s11557-016-1248-y
Réblová M, Miller AN, Réblová K, Štěpánek V (2018) Phylogenetic classification and generic delineation of Calyptosphaeria gen. nov., Lentomitella, Spadicoides and Torrentispora (Sordariomycetes). Stud Mycol 89:1–62. https://doi.org/10.1016/j.simyco.2017.11.004
Réblová M, Hernández-Restrepo M, Fournier J, Nekvindová J (2020a) New insights into the systematics of Bactrodesmium and its allies and introducing new genera, species and morphological patterns in the Pleurotheciales and Savoryellales (Sordariomycetes). Stud Mycol 95:415–466. https://doi.org/10.1016/j.simyco.2020.02.002
Réblová M, Nekvindová J, Fournier J, Miller AN (2020b) Delimitation, new species and teleomorph-anamorph relationships in Codinaea, Dendrophoma, Paragaeumannomyces and Striatosphaeria (Chaetosphaeriaceae). MycoKeys 74:17–74. https://doi.org/10.3897/mycokeys.74.57824
Réblová M, Nekvindová J, Kolařik M, Hernández-Restrepo M (2021a) Delimination and phylogeny of Dictyochaeta, and introduction of Archochaeta and Tubulicolla, genera nova. Mycologia 113:390–433. https://doi.org/10.1080/00275514.2020.1822095
Réblová M, Kolařik M, Nekvindová J, Miller AN, Hernández-Restrepo M (2021b) Phylogeny, global biogrography and pleomorphism of Zanclospora. Microorganism 9:706. https://doi.org/10.3390/microorganisms9040706
Réblová M, Nekvindová J, Hernández-Restrepo M (2021c) Reflections on Menisporopsis, Multiguttulispora and Tainosphaeria using molecular and morphological data. J Fungi 7(6):438. https://doi.org/10.3390/jof7060438
Réblová M, Nekvindová J, Miller AN (2021d) Phylogeny and taxonomy of Catenularia and similar fungi with catenate conidia. MycoKeys 81:1–44. https://doi.org/10.3897/mycokeys.81.67785
Réblová M, Kolařík M, Nekvindová J, Réblová J, Sklenář F, Miller AN, Hernández-Restrepo M (2021e) Phylogenetic reassessment, taxonomy, and biogeography of Codinaea and similar fungi. J Fungi 7(12):1097. https://doi.org/10.3390/jof7121097
Reeves WK (2000) Caecidotea carolinensis (Isopoda: Asellidae): first record of a stygobite from South Carolina. J Cave Karst Stud 62(1):18–19
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98. https://doi.org/10.3852/mycologia.97.1.84
Rehner SA, Samuels GJ (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol Res 98:625–634. https://doi.org/10.1016/s0953-7562(09)80409-7
Rifai MA (1965) On Sporidesmium trigonellum Sacc. Persoonia 3(4):407–411
Rossman AY, Allen WC, Braun U, Castlebury LA, Chaverri P, Crous PW, Hawksworth DL, Hyde KD, Johnston P, Lombard L, Romberg M, Samson RA, Seifert KA, Stone JK, Udayanga D, White JF (2016) Overlooked competing asexual and sexually typified generic names of Ascomycota with recommendations for their use or protection. IMA Fungus 7(2):289–308. https://doi.org/10.5598/imafungus.2016.07.02.09
Saccardo PA (1883) Sylloge Pyrenomycetum, vol II. Sylloge Fungorum 2:1–813
Samarakoon MC, Hyde KD, Promputtha I, Hongsanan S, Ariyawansa HA, Maharachchikumbura SSN, Daranagama DA, Stadler M, Mapook A (2016) Evolution of Xylariomycetidae (Ascomycota: Sordariomycetes). Mycosphere 7(11):1746–1761. https://doi.org/10.5943/mycosphere/7/11/9
Samarakoon MC, Liu JK, Hyde KD, Promputtha I (2019) Two new species of Amphisphaeria (Amphisphaeriaceae) from northern Thailand. Phytotaxa 391:207–217. https://doi.org/10.11646/phytotaxa.391.3.4
Samarakoon MC, Maharachchikumbura SSN, Liu JK, Hyde KD, Promputtha I, Stadler M (2020) Molecular phylogeny and morphology of Amphisphaeria (= Lepteutypa) (Amphisphaeriaceae). J Fungi 6(3):174. https://doi.org/10.3390/jof6030174
Samuels GJ, Müller E (1978) Life-history studies of Brazilian Ascomycetes. 1. Two new genera of the Sphaeriaceae having, respectively, Sporoschisma-like and Codinaea anamorphs. Sydowia 31:126–136. https://doi.org/10.1007/bf02464321
Sati SC, Belwal M, Pargaien N (2008) Diversity of water borne conidial fungi as root endophytes in temperate forest of western Himalaya. Nat Sci 6:59–69
Seifert KA, Morgan-Jones G, Gams W, Kendrick B (2011) The genera of hyphomycetes. CBS-KNAW Fungal Biodiversity Centre, Utrecht. https://doi.org/10.3767/003158511X617435
Senanayake IC, Maharachchikumbura SSN, Hyde KD, Bhat JD, Jones EBG, McKenzie EHC, Dai DQ, Daranagama DA, Dayarathne MC, Goonasekara ID, Konta S, Li WJ, Shang QJ, Stadler M, Wijayawardene NN, Xiao YP, Norphanphoun C, Li Q, Liu XZ, Bahkali AH, Kang JC, Wang Y, Wen TC, Wendt L, Xu JC, Camporesi E (2015) Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers 73:73–144. https://doi.org/10.5943/mycosphere/7/11/9
Senanayake IC, Lian TT, Mai XM, Camporesi E, Zeng YJ, Tian SL, Xie N (2019) Taxonomy and phylogeny of Amphisphaeria acericola sp. nov from Italy. Phytotaxa 403:285–292. https://doi.org/10.11646/phytotaxa.403.4.2
Serrano L, Sosa D, Magdama F, Espinoza F, Quevedo A, Vera M, Villavicencio M, Maridueña G, Pérez-Martinez S, Malosso E, Ramos-García B, Castañeda-Ruiz RF (2020) Neomyrmecridium asymmetricum sp. nov. from Ecuador. Mycotaxon 135:151–165. https://doi.org/10.5248/135.151
Shearer CA (1973) Fungi of the Chesapeake Bay and its Tributaries II. The Genus Conioscypha. Mycologia 65:128–136. https://doi.org/10.2307/3757793
Shearer CA (1989) Pseudohalonectria (Lasiosphaeriaceae), an antagonistic genus from wood in freshwater. Can J Bot 67:1944–1955. https://doi.org/10.1139/b89-247
Shearer CA (1993) The freshwater ascomycetes. Nova Hedwig 56:1–33
Shearer CA, Crane JL (1995) Boerlagiomyces websteri, a new ascomycete from fresh water. Mycologia 87:876–879. https://doi.org/10.2307/3760863
Shearer CA, Motta JJ (1973) Ultrastructure and conidiogenesis in Conioscypha (Hyphomycetes). Can J Bot 51:1747–1751. https://doi.org/10.1139/b73-226
Shearer CA, Crane JL, Chen W (1999) Freshwater Ascomycetes: Ophioceras species. Mycologia 91:145–156. https://doi.org/10.2307/3761204
Shearer CA, Langsam DM, Longcore JE (2004) Fungi in freshwater habitats. In: Mueller GM, Bills GF, Foster MS (eds) Measuring and monitoring biological diversity: standard methods for fungi. Smithsonian Institution Press, Washington, pp 513–531
Shen HW, Bao DF, Su HY, Boonmee S, Luo ZL (2021) Rostriconidium cangshanense sp. nov. and a new record R. pandanicola (Torulaceae) in Yunnan Province, China. Mycosystema 40(6):1259–1273. https://doi.org/10.13346/j.mycosystema.200306
Shenoy BD, Jeewon R, Wu WP, Bhat DJ, Hyde KD (2006) Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycol Res 110:916–928. https://doi.org/10.1016/j.mycres.2006.06.004
Shirouzu T, Harada Y (2004) Notes on species of Helminthosporium and its allied genera in Japan. Mycoscience 45:17–23. https://doi.org/10.1007/s10267-003-0146-8
Simms MJ (2014) Karst and paleokarst. In: Reference module in earth systems and environmental sciences. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-409548-9.09370-2
Sivanesan A, Alcorn JL (2002) Australiasca queenslandica gen. et sp. nov. (Chaetosphaeriaceae: Ascomycota) and its anamorph Dischloridium camelliae sp. nov. from Australia. Aust J Bot 15:741–747. https://doi.org/10.1071/sb01049
Sivichai S, Hywel-Jones N, Jones EBG (1998) Lignicolous freshwater Ascomycota from Thailand: 1. Ascotaiwania sawadae and its anamorph state Monotosporella. Mycoscience 39:307–311. https://doi.org/10.1007/bf02464013
Sivichai S, Hywel-Jones NL, Somrithipol S (2000) Lignicolous freshwater Ascomycota from Thailand: Melanochaeta and Sporoschisma anamorphs. Mycol Res 104:478–485. https://doi.org/10.1017/s0953756299001604
Smith GJD, Liew ECY, Hyde KD (2003) The Xylariales: a monophyletic order containing 7 families. Fungal Divers 13:185–218
Soares DJ, Barreto RW (2008) Fungal pathogens of the invasive riparian weed Hedychium coronarium from Brazil and their potential for biological control. Fungal Divers 28:85–96. https://doi.org/10.1071/DN08034
Somrithipol S, Chatmala I, Jones EBG (2002) Cirrenalia nigrospora sp. nov. and C. tropicalis from Thailand. Nova Hedwig 75:477–485. https://doi.org/10.1127/0029-5035/2002/0075-0477
Sri-indrasutdhi V, Boonyuen N, Suetrong S, Chuaseeharonnachai C, Sivichai S, Jones EBG (2010) Wood-inhabiting freshwater fungi from Thailand: Ascothailandia grenadoidia gen. et sp. nov., Canalisporium grenadoidia sp. nov. with a key to Canalisporium species (Sordariomycetes, Ascomycota). Mycoscience 51:411–420. https://doi.org/10.1007/s10267-010-0055-6
Sridhar KR, Arun AB, Maria GL, Madhyastha MN (2011) Diversity of fungi on submerged leaf and woody litter in River Kali, Southwest India. Environ Res J 5:1–14
Stamatakis A (2014) RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Stanley SJ, Hyde KD (1997) Boerlagiomyces grandisporus sp. nov., a new tropical freshwater ascomycete from the Philippines. Mycol Res 101(5):635–640. https://doi.org/10.1017/s0953756296003103
Su H, Hyde KD, Maharachchikumbura SSN, Ariyawansa HA, Luo Z, Promputtha I, Tian Q, Lin C, Shang Q, Zhao Y, Chai H, Liu X, Bahkali AH, Bhat JD, McKenzie EHC, Zhou D (2016a) The families Distoseptisporaceae fam. nov., Kirschsteiniotheliaceae, Sporormiaceae and Torulaceae, with new species from freshwater in Yunnan Province, China. Fungal Divers 80:375–409. https://doi.org/10.1007/s13225-016-0362-0
Su HY, Luo ZL, Liu XY, Su XJ, Hu DM, Zhou DQ, Bahkali AH, Hyde KD (2016b) Lentithecium cangshanense sp. nov. (Lentitheciaceae) from freshwater habitats in Yunnan Province, China. Phytotaxa 267(1):61–69. https://doi.org/10.11646/phytotaxa.267.1.6
Su XJ, Luo ZL, Jeewon R, Bhat DJ, Bao DF, Li WL, Hao YE, Su HY, Hyde KD (2018) Morphology and multigene phylogeny reveal new genus and species of Torulaceae from freshwater habitats in northwestern Yunnan, China. Mycol Prog 17:531–545. https://doi.org/10.1007/s11557-018-1388-3
Subramanian CV (1992) A reassessment of Sporidesmium (hyphomycetes) and some related taxa. Proc Indian Natn Sci Acad B58(4):179–190
Subramanian CV, Bhat DJ (1987) Hyphomycetes from south India I. some new taxa. Kavaka 15:41–74
Sudheep NM, Sridhar KR (2011) Diversity of lignicolous and Ingoldian fungi on woody litter from the river Kali (Western Ghats, India). Mycology 2:98–108. https://doi.org/10.1080/21501203.2011.554905
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG (2009) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173. https://doi.org/10.3114/sim.2009.64.09
Sun LY, Li HY, Sun X, Guo LD (2017) Dematipyriforma aquilaria gen. et sp. nov., a new hyphomycetous taxon from Aquilaria crassna. Cryptogam Mycol 38:341–351. https://doi.org/10.7872/crym/v38.iss3.2017.341
Sun YR, Jayawardena RS, Hyde KD, Wang Y (2021) Kirschsteiniothelia thailandica sp. nov. (Kirschsteiniotheliaceae) from Thailand. Phytotaxa 490(2):172–182. https://doi.org/10.11646/phytotaxa.490.2.3
Sutton BC (1973) Hyphomycetes from Manitoba and Saskatchewan. Mycol Pap 132:1–143
Tanaka K, Harada Y (2003) Pleosporales in Japan (3). The genus Massarina. Mycoscience 44:173–185. https://doi.org/10.1007/s10267-003-0102-7
Tanaka K, Harada Y (2004) Pleosporales in Japan (4). The genus Massariosphaeria. Mycoscience 45:96–105. https://doi.org/10.1007/s10267-003-0160-x
Tanaka K, Harada Y, Barr ME (2005) Bambusicolous fungi in Japan (3): a new combination, Kalmusia scabrispora. Mycoscience 46:110–113. https://doi.org/10.1007/s10267-004-0224-6
Tanaka K, Hirayama K, Yonezawa H, Hatakeyama S, Harada Y, Sano T, Shirouzu T, Hosoya T (2009) Molecular taxonomy of bambusicolous fungi: Tetraplosphaeriaceae, a new pleosporalean family with tetraploa-like anamorphs. Stud Mycol 64:175–209. https://doi.org/10.3114/sim.2009.64.10
Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y (2015) Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud Mycol 82:75–136. https://doi.org/10.1016/j.simyco.2015.10.002
Tang A, Jeewon R, Hyde K (2009) A re-evaluation of the evolutionary relationships within the Xylariaceae based on ribosomal and protein-coding gene sequences. Fungal Divers 34:127–155
Teng SC (1936) Additional Fungi from China. II. Sinensia 7:490–527
Thambugala KM, Wanasinghe DN, Phillips AJL, Camporesi E, Bulgakov TS, Phukhamsakda C, Ariyawansa HA, Goonasekara ID, Phookamsak R, Dissanayake A (2017) Mycosphere notes 1–50: grass (Poaceae) inhabiting Dothideomycetes. Mycosphere 8:697–796. https://doi.org/10.5943/mycosphere/8/4/13
Thomas K (1996) Freshwater fungi. In: Orchard AE (ed) Fungi of Australia 1B, Introduction—Fungi in the Environment. Australian Biological Resources Study, Canberra, pp 1–37
Thongkantha S, Jeewon R, Vijaykrishna D, Lumyong S, McKenzie E, Hyde K (2009) Molecular phylogeny of Magnaporthaceae (Sordariomycetes) with a new species Ophioceras chiangdaoense from Dracaena loureiroi in Thailand. Fungal Divers 34:157–173
Tian Q, Doilom M, Luo ZL, Chomnunti P, Bhat JD, Xu JC, Hyde KD (2016) Introducing Melanoctona tectonae gen. et sp. nov. and Minimelanolocus yunnanensis sp. nov. (Herpotrichiellaceae, Chaetothyriales). Cryptogam Mycol 37:477–492. https://doi.org/10.7872/crym/v37.iss4.2016.477
Tian Q, Chomnunti P, Lumyong S, Liu JK, Hyde KD (2021) Phylogenetic relationships and morphological reappraisal of Chaetothyriales. Mycosphere 12(1):1157–1261. https://doi.org/10.5943/mycosphere/12/1/15
Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SSN, Liu JK, Bhat DJ, Jones EBG, McKenzie EHC, Camporesi E, Bulgakov TS, Doilom M, de Azevedo SALCM, Das K, Manimohan P, Gibertoni TB, Lim YW, Ekanayaka AH, Thongbai B, Lee HB, Yang JB, Kirk PM, Sysouphanthong P, Singh SK, Boonmee S, Dong W, Raj KNA, Latha KPD, Phookamsak R, Phukhamsakda C, Konta S, Jayasiri SC, Norphanphoun C, Tennakoon DS, Li J, Dayarathne MC, Perera RH, Xiao Y, Wanasinghe DN, Senanayake IC, Goonasekara ID, de Silva NI, Mapook A, Jayawardena RS, Dissanayake AJ, Manawasinghe IS, Chethana KWT, Luo ZL, Hapuarachchi KK, Baghela A, Soares AM, Vizzini A, Meiras-Ottoni A, Mešić A, Dutta AK, de Souza CAF, Richter C, Lin CG, Chakrabarty D, Daranagama DA, Lima DX, Chakraborty D, Ercole E, Wu F, Simonini G, Vasquez G, da Silva GA, Plautz HL, Ariyawansa HA, Lee H, Kušan I, Song J, Sun J, Karmakar J, Hu K, Semwal KC, Thambugala KM, Voigt K, Acharya K, Rajeshkumar KC, Ryvarden L, Jadan M, Hosen MI, Mikšík M, Samarakoon MC, Wijayawardene NN, Kim NK, Matočec N, Singh PN, Tian Q, Bhatt RP, de Oliveira RJV, Tulloss RE, Aamir S, Kaewchai S, Marathe SD, Khan S, Hongsanan S, Adhikari S, Mehmood T, Bandyopadhyay TK, Svetasheva TY, Nguyen TTT, Antonín V, Li WJ, Wang Y, Indoliya Y, Tkalčec Z, Elgorban AM, Bahkali AH, Tang AMC, Su HY, Zhang H, Promputtha I, Luangsa-ard J, Xu J, Yan J, Ji-Chuan K, Stadler M, Mortimer PE, Chomnunti P, Zhao Q, Phillips AJL, Nontachaiyapoom S, Wen TC, Karunarathna SC (2017) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261. https://doi.org/10.1007/s13225-017-0378-0
Tibpromma S, Hyde KD, McKenzie EHC, Bhat DJ, Phillips AJL, Wanasinghe DN, Samarakoon MC, Jayawardena RS, Dissanayake AJ, Tennakoon DS, Doilom M, Phookamsak R, Tang AMC, Xu J, Mortimer PE, Promputtha I, Maharachchikumbura SSN, Khan S, Karunarathna SC (2018) Fungal diversity notes 840–928: micro-fungi associated with Pandanaceae. Fungal Divers 93:1–160. https://doi.org/10.1007/s13225-018-0408-6
Toofanee SB, Dulymamode R (2002) Fungal endophytes associated with Cordemoya integrofolia. Fungal Divers 11:169–175
Tóth S (2009) Boerlagiomyces websteri (Ascomycota, Tubeufiaceae) from Hungary, first record outside the USA. Mycol Balc 6:85–86
Tsang CC, Chan JFW, Trendell-Smith NJ, Ngan AHY, Ling IWH, Lau SKP, Woo PCY (2014) Subcutaneous phaeohyphomycosis in a patient with IgG4-related sclerosing disease caused by a novel ascomycete, Hongkongmyces pedis gen. et sp. nov.: first report of human infection associated with the family Lindgomycetaceae. Med Mycol J 52:736–747. https://doi.org/10.1093/mmy/myu043
Tsui CKM, Berbee ML (2006) Phylogenetic relationships and convergence of helicosporous fungi inferred from ribosomal DNA sequences. Mol Phylog Evol 39:587–597. https://doi.org/10.1016/j.ympev.2006.01.025
Tsui CKM, Goh TK, Hyde KD (1997) A new species of Dactylaria from Hong Kong. Sydowia 49:182–186
Tsui CKM, Goh TK, Hyde KD, Hodgkiss IJ (1999) Reflections on Menisporopsis, with the addition of M. multisetulata sp. nov. from submerged wood in Hong Kong. Mycol Res 103:148–152. https://doi.org/10.1017/s0953756298006923
Tsui CKM, Goh TK, Hyde KD (2001a) A revision of the genus Exserticlava, with a new species. Fungal Divers 7:135–143
Tsui CKM, Leung YM, Hyde KD, Hodgkiss IJ (2001b) Three new Ophioceras species (Ascomycetes) from the tropics. Mycoscience 42:321–326. https://doi.org/10.1007/bf02461213
Tsui CKM, Hyde KD, Hodgkiss IJ (2001c) Paraniesslia tuberculata gen. et sp. nov., and new records or species of Clypeosphaeria, Leptosphaeria and Astrosphaeriella in Hong Kong freshwater habitats. Mycologia 93:1002–1009. https://doi.org/10.2307/3761763
Tsui CKM, Ranghoo VM, Hodgkiss IJ, Hyde KD (2002) Three new species of Annulatascus (Ascomycetes) from Hong Kong freshwater habitats. Mycoscience 43:383–389. https://doi.org/10.1007/s102670200056
Tsui CKM, Goh TK, Hyde KD (2003) Reflections on the genus Vanakripa, and a description of V. ellipsoidea sp. nov. Mycologia 95:124–127. https://doi.org/10.2307/3761970
Udagawa SI, Toyazaki N (1983) A new species of Conioscypha. Mycotaxon 18:131–137
Untereiner WA (2000) Capronia and its anamorphs: exploring the value of morphological and molecular characters in the systematics of the Herpotrichiellaceae. Stud Mycol 45:141–148
Untereiner WA, Naveau FA (1999) Molecular systematics of the Herpotrichiellaceae with an assessment of the phylogenetic positions of Exophiala dermatitidis and Phialophora Americana. Mycologia 91:67–83. https://doi.org/10.2307/3761194
Vaidya G, Lohman DJ, Meier R (2011) SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27:171–180. https://doi.org/10.1111/j.1096-0031.2010.00329.x
Vijaykrishna D, Jeewon R, Hyde KD (2005) Fusoidispora aquatica: a new freshwater ascomycete from Hong Kong based on morphology and phylogeny inferred from rDNA gene sequences. Sydowia 57:267–280
Vijaykrishna D, Jeewon R, Hyde KD (2006) Molecular taxonomy, origins and evolution of freshwater ascomycetes. Fungal Divers 23:351–390
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
Vittal BPR (1975) Gangliostilbe indica, a new synnematous hyphomycetes from India. Kavaka 3:69–71
von Arx JA (1971) Testudinaceae, a new family of ascomycetes. Persoonia 6:365–369
von Arx JA, Müller E (1975) A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Stud Mycol 9:1–159. https://doi.org/10.2307/3758851
von Höhnel F (1909) Fragmente zur Mykologie (VI. Mitteilung, Nr. 182 bis 288), gleichzeitg Zweite Mitteilung über die Ergebnisse der mit Unterstützung der kaiserl. Akademie 1907–1908 von ihm ausgeführten Forschungsreise nach Java. Sitzungsberichte der Akademie der Wissenschaften mathematisch-naturwissenschaftliche Klasse 118:275–452
Vu D, Groenewald M, de Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T, Crous PW, Robert V, Verkley GJM (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom Fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud Mycol 92:135–154. https://doi.org/10.1016/j.simyco.2018.05.001
Walker J (1980) Gaeumannomyces, Linocarpon, Ophiobolus and several other genera ofscolecos pored Ascomycetes and Phialophora conidial states, with a note on hyphopodia. Mycotaxon 11:1–129
Wan YL, Bao DF, Luo ZL, Bhat DJ, Xu YX, Su HY, Hao YE (2021) Two new species of Minimelanolocus (Herpotrichiellaceae, Chaetothyriales) from submerged wood in Yunnan, China. Phytotaxa 480(1):45–56. https://doi.org/10.11646/phytotaxa.480.1.4
Wanasinghe DN, Jones EBG, Camporesi E, Boonmee S, Ariyawansa HA, Wijayawardene NN, Mortimer PE, Xu J, Yang JB, Hyde KD (2014) An exciting novel member of Lentitheciaceae in Italy from Clematis Vitalba. Cryptogam Mycol 35:323–337. https://doi.org/10.7872/crym.v35.iss4.2014.323
Wanasinghe DN, Jones EBG, Camporesi E, Mortimer PE, Xu JC, Bahkali AH, Hyde KD (2015) The genus Murispora. Cryptogam Mycol 36:419–448. https://doi.org/10.7872/crym/v36.iss4.2015.419
Wanasinghe DN, Jeewon R, Tibpromma S, Jones EBG, Hyde KD (2017) Saprobic Dothideomycetes in Thailand: Muritestudina gen. et sp. nov. (Testudinaceae) a new terrestrial pleosporalean ascomycete, with hyaline and muriform ascospores. Stud Fungi 2:219–234. https://doi.org/10.5943/sif/2/1/26
Wanasinghe DN, Jeewon R, Jones EBG, Boonmee S, Kaewchai S, Manawasinghe IS, Lumyong S, Hyde KD (2018a) Novel palmicolous taxa within Pleosporales: multigene phylogeny and taxonomic circumscription. Mycol Prog 17:571–590. https://doi.org/10.1007/s11557-018-1379-4
Wanasinghe DN, Phukhamsakda C, Hyde KD, Jeewon R, Lee HB, Gareth Jones EB, Tibpromma S, Tennakoon DS, Dissanayake AJ, Jayasiri SC, Gafforov Y, Camporesi E, Bulgakov TS, Ekanayake AH, Perera RH, Samarakoon MC, Goonasekara ID, Mapook A, Li WJ, Senanayake IC, Li J, Norphanphoun C, Doilom M, Bahkali AH, Xu J, Mortimer PE, Tibell L, Tibell S, Karunarathna SC (2018b) Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers 89:1–236. https://doi.org/10.1007/s13225-018-0395-7
Wang CJK, Sutton BC (1982) New and rare lignicolous Hyphomycetes. Mycologia 74:489–500. https://doi.org/10.2307/3792971
Wang YZ, Aptroot A, Hyde KD (2004) Revision of the Ascomycete Genus Amphisphaeria. Fungal Divers Res Ser 13:1–168
Wang GN, Yu XD, Dong W, Bhat DJ, Boonmee S, Zhang D, Zhang H (2019) Freshwater hyphomycetes in Eurotiomycetes: a new species of Minimelanolocus and a new collection of Thysanorea papuana (Herpotrichiellaceae). Mycol Prog 18:511–522. https://doi.org/10.1007/s11557-019-01473-7
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322. https://doi.org/10.1016/b978-0-12-372180-8.50042-1
Whitton SR, McKenzie EHC, Hyde KD (2012) Fungi associated with Pandanaceae. Fungal Divers Res Ser 21:1–410. https://doi.org/10.1007/978-94-007-4447-9_4
Wijayawardene NN, Hyde KD, Bhat DJ, Goonasekara ID, Nadeeshan D, Camporesi E, Schumacher RK, Wang Y (2015) Additions to brown-spored coelomycetous taxa in Massarinae, Pleosporales: introducing Phragmocamarosporium gen. nov. and Suttonomyces gen. nov. Cryptogam Mycol 36:213–224. https://doi.org/10.7872/crym/v36.iss2.2015.213
Wijayawardene NN, Hyde KD, Lumbsch HT, Liu JK, Maharachchikumbura SSN, Ekanayaka AH, Tian Q, Phookamsak R (2018) Outline of Ascomycota: 2017. Fungal Divers 88:167–263. https://doi.org/10.1007/s13225-018-0394-8
Wijayawardene NN, Hyde KD, Al-Ani LKT, Tedersoo L, Haelewaters D, Rajeshkumar KC, Zhao RL, Aptroot A, Leontyev DV, Saxena RK, Tokarev YS, Dai DQ, Letcher PM, Stephenson SL, Ertz D, Lumbsch HT, Kukwa M, Issi IV, Madrid H, Phillips AJL, Selbmann L, Pfliegler WP, Horváth E, Bensch K, Kirk PM, Kolaříková K, Raja HA, Radek R, Papp V, Dima B, Ma J, Malosso E, Takamatsu S, Rambold G, Gannibal PB, Triebel D, Gautam AK, Avasthi S, Suetrong S, Timdal E, Fryar SC, Delgado G, Réblová M, Doilom M, Dolatabadi S, Pawłowska J, Humber RA, Kodsueb R, Sánchez-Castro I, Goto BT, Silva DKA, de Souza FA, Oehl F, da Silva GA, Silva IR, Błaszkowski J, Jobim K, Maia LC, Barbosa FR, Fiuza PO, Divakar PK, Shenoy BD, Castañeda-Ruiz RF, Somrithipol S, Lateef AA, Karunarathna SC, Tibpromma S, Mortimer PE, Wanasinghe DN, Phookamsak R, Xu J, Wang Y, Tian F, Alvarado P, Li DW, Kušan I, Matočec N, Maharachchikumbura SSN, Papizadeh M, Heredia G, Wartchow F, Bakhshi M, Boehm E, Youssef N, Hustad VP, Lawrey JD, Santiago ALCMA, Bezerra JDP, Souza-Motta CM, Firmino AL, Tian Q, Houbraken J, Hongsanan S, Tanaka K, Dissanayake AJ, Monteiro JS, Grossart HP, Suija A, Weerakoon G, Etayo J, Tsurykau A, Vázquez V, Mungai P, Damm U, Li QR, Zhang H, Boonmee S, Lu YZ, Becerra AG, Kendrick B, Brearley FQ, Motiejūnaitė J, Sharma B, Khare R, Gaikwad S, Wijesundara DSA, Tang LZ, He MQ, Flakus A, Rodriguez-Flakus P, Zhurbenko MP, McKenzie EHC, Stadler M, Bhat DJ, Liu JK, Raza M, Jeewon R, Nassonova ES, Prieto M, Jayalal RGU, Erdoğdu M, Yurkov A, Schnittler M, Shchepin ON, Novozhilov YK, SilvaFilho AGS, Liu P, Cavender JC, Kang Y, Mohammad S, Zhang LF, Xu RF, Li YM, Dayarathne MC, Ekanayaka AH, Wen TC, Deng CY, Pereira OL, Navathe S, Hawksworth DL, Fan XL, Dissanayake LS, Kuhnert E, Grossart HP, Thines M (2020) Outline of Fungi and fungus-like taxa. Mycosphere 11:1060–1456. https://doi.org/10.5943/mycosphere/11/1/8
Wijayawardene NN, Hyde KD, Dai DQ, Sánchez-García M, Goto BT, Saxena RK, Erdoğdu M, Selçuk F, Rajeshkumar KC, Aptroot A, Błaszkowski J, Boonyuen N, da Silva GA, de Souza FA, Dong W, Ertz D, Haelewaters D, Jones EBG, Karunarathna SC, Kirk PM, Kukwa M, Kumla J, Leontyev DV, Lumbsch HT, Maharachchikumbura SSN, Marguno F, Martínez-Rodríguez P, Mešić A, Monteiro JS, Oehl F, Pawłowska J, Pem D, Pfliegler WP, Phillips AJL, Pošta A, He MQ, Li JX, Raza M, Sruthi OP, Suetrong S, Suwannarach N, Tedersoo L, Thiyagaraja V, Tibpromma S, Tkalčec Z, Tokarev YS, Wanasinghe DN, Wijesundara DSA, Wimalaseana SDMK, Madrid H, Zhang GQ, Gao Y, Sánchez-Castro I, Tang LZ, Stadler M, Yurkov A, Thines M (2022) Outline of Fungi and fungus-like taxa—2021. Mycosphere 13(1):53–453. https://doi.org/10.5943/mycosphere/13/1/2
Wong SW, Hyde KD, Jones EBG (1998a) Annulatascaceae, a new ascomycete family from the tropics. Systema Ascomycetum 16:17–25
Wong MKM, Goh TK, Hodgkiss IJ, Hyde KD, Ranghoo VM, Tsui CKM, Ho WH, Wong WSW, Yuen TK (1998b) Role of fungi in freshwater ecosystems. Biol Conserv 7:1187–1206. https://doi.org/10.1023/A:1008883716975
Woudenberg JHC, Groenewald JZ, Binder M, Crous PW (2013) Alternaria redefined. Stud Mycol 75:171–212. https://doi.org/10.3114/sim0015
Wu WP, Diao YZ (2022) Anamorphic chaetosphaeriaceous fungi from China. Fungal Divers 116:1–546. https://doi.org/10.21203/rs.3.rs-1253155/v1
Wu WP, Zhuang WY (2005) Sporidesmium, Endophragmiella and related genera from China. Fungal Divers Res Ser 15:1–351
Wurzbacher C, Kerr J, Grossart HP (2011) Aquatic fungi. In: Grillo O, Venora G (eds) The dynamical processes of biodiversity—case studies of evolution and spatial distribution. InTech, Rijeka, pp 227–258. https://doi.org/10.5772/1830
Wurzbacher C, Rösel S, Rychła A, Grossart HP (2014) Importance of saprotrophic freshwater fungi for pollen degradation. PLoS ONE 9:e94643. https://doi.org/10.1371/journal.pone.0094643
Xia JW, Ma YR, Li Z, Zhang XG (2017) Acrodictys-like wood decay fungi from southern China, with two new families Acrodictyaceae and Junewangiaceae. Sci Rep 7:7888. https://doi.org/10.1038/s41598-017-08318-x
Xie L, Chen YL, Long YY, Zhang Y, Liao ST, Liu B, Qin LP, Nong Q, Zhang WL (2019) Three new species of Conlarium from sugarcane rhizosphere in southern China. MycoKeys 56:1–11. https://doi.org/10.3897/mycokeys.56.35857
Yang J, Liu JK, Hyde KD, Bhat DJ, Jones EBG, Liu ZY (2016a) New species of Sporoschisma (Chaetosphaeriaceae) from aquatic habitats in Thailand. Phytotaxa 289:147–157. https://doi.org/10.11646/phytotaxa.289.2.4
Yang J, Maharachchikumbura SSN, Bhat DJ, Hyde KD, McKenzie EHC, Jones EBG, Al-Sadi AM, Lumyong S (2016b) Fuscosporellales, a new order of aquatic and terrestrial Hypocreomycetidae (Sordariomycetes). Cryptogam Mycol 37:449–475. https://doi.org/10.7872/crym/v37.iss4.2016.449
Yang J, Liu JK, Hyde KD, Jones EBG, Liu ZY (2017) Two new species in Fuscosporellaceae from freshwater habitats in Thailand. Mycosphere 8(10):1893–1903. https://doi.org/10.5943/mycosphere/8/10/12
Yang J, Maharachchikumbura SSN, Liu JK, Hyde KD, Jones EBG, Al-Sadi AM, Liu ZY (2018a) Pseudostanjehughesia aquitropica gen. et sp. nov. and Sporidesmium sensu lato species from freshwater habitats. Mycol Prog 17:591–616. https://doi.org/10.1007/s11557-017-1339-4
Yang J, Liu JK, Hyde KD, Jones EBG, Liu ZY (2018b) New species in Dictyosporium, new combinations in Dictyocheirospora and an updated backbone tree for Dictyosporiaceae. Mycokeys 36:83–105. https://doi.org/10.3897/mycokeys.36.27051
Yang J, Liu NG, Liu JK, Hyde KD, Jones EBG, Liu ZY (2018c) Phylogenetic placement of Cryptophiale, Cryptophialoidea, Nawawia, Neonawawia gen. nov. and Phialosporostilbe. Mycosphere 9(6):1132–1150. https://doi.org/10.5943/mycosphere/9/6/5
Yang H, Dong W, Yu XD, Bhat DJ, Boonmee S, Zhang H (2020) Four freshwater dematiaceous hyphomycetes in Sordariomycetes with two new species of Parafuscosporella. Phytotaxa 441:19–34. https://doi.org/10.11646/phytotaxa.441.1.2
Yuan ZL, Rao LB, Chen YC, Zhang CL, Wu YG (2011) From pattern to process: species and functional diversity in fungal endophytes of Abies beshanzuensis. Fungal Biol 115:197–213. https://doi.org/10.1016/j.funbio.2010.11.002
Yuan HS, Lu X, Dai YU, Hyde KD, Kan YH, Kušan I, He SH, Liu N, Sarma VV, Zhao CL, Cui BK, Yousaf N, Sun G, Liu SY, Wu F, Lin CG, Dayarathne MC, Gibertoni TB, Conceição LB, GaribayOrijel R, Villegas-Ríos M, Salas-Lizana R, Wei TZ, Qiu JZ, Yu ZF, Phookamsak R, Zeng M, Paloi S, Bao DF, Abeywickrama PD, Wei DP, Jing Y, Manawasinghe IS, Harishchandra D, Brahmanage RS, de Silva NI, Tennakoon DS, Karunarathna A, da Silva GA, Gafforov Y, Pem D, Zhang S, de Santiago ALCM, Bezerra JDP, Dima B, Acharya K, Alvarez-Manjarrez J, Bahkali AH, Bhatt VK, Brandrud TE, Bulgakov TS, Camporesi E, Cao T, Chen YX, Chen YY, Devadatha B, Elgorban AM, Fan LF, Du X, Gao L, Gonçalves CM, Gusmão LFP, Huanraluek N, Jadan M, Jayawardena RS, Khalid AN, Langer E, Lima DX, de Lima-Júnior NC, de Lira CRS, Liu JK, Liu S, Lumyong S, Luo ZL, Matočec N, Niranjan M, Oliveira-Filho JRC, Papp V, PérezPazos E, Phillips AJL, Qiu PL, Ren Y, Ruiz RFC, Semwal KC, da Silva RMF, Soop K, de Souza CAF, Souza-Motta CM, Sun LH, Xie ML, Yao YJ, Zhao Q, Zhou LW (2020) Fungal diversity notes 1277–1386: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 104:1–266. https://doi.org/10.1007/s13225-020-00461-7
Zelski SE, Raja HA, Miller AN, Shearer CA (2011) Chaetorostrum quincemilensis, gen. et sp. nov., a new freshwater ascomycete and its Taeniolella-like anamorph from Peru. Mycosphere 2(5):593–600. https://doi.org/10.5943/mycosphere/2/5/9/
Zelski SE, Balto JA, Do C, Raja HA, Miller AN, Shearer CA (2014) Phylogeny and morphology of dematiaceous freshwater microfungi from Perú. IMA Fungus 5:425–438. https://doi.org/10.5598/imafungus.2014.05.02.07
Zelski SE, Raja HA, Miller AN, Shearer CA (2015) Conioscypha peruviana sp. nov., its phylogenetic placement based on 28S rRNA gene, and a report of Conioscypha gracilis from Peru. Mycoscience 56:319–325. https://doi.org/10.1016/j.myc.2014.09.002
Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung GH (2006) An overview of the systematics of the Sordariomycetes based on four-gene phylogeny. Mycologia 98:1077–1088. https://doi.org/10.3852/mycologia.98.6.1076
Zhang Y, Jeewon R, Fournier J, Hyde KD (2008) Multi-gene phylogeny and morphotaxonomy of Amniculicola lignicola: a novel freshwater fungus from France and its relationships to the Pleosporales. Mycol Res 112:1186–1194. https://doi.org/10.1016/j.mycres.2008.04.004
Zhang Y, Wang HK, Fournier J, Crous PW, Jeewon R, Pointing SB, Hyde KD (2009a) Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Divers 38:225–251
Zhang K, Ma LG, Zhang XG (2009b) New species and records of Shrungabeeja from southern China. Mycologia 101(4):573–578. https://doi.org/10.3852/09-006
Zhang Y, Schoch CL, Fournier J, Crous PW, De Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Hyde KD (2009c) Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102. https://doi.org/10.3114/sim.2009.64.04
Zhang Y, Fournier J, Crous PW, Pointing SB, Hyde KD (2009d) Phylogenetic and morphological assessment of two new species of Amniculicola and their allies (Pleosporales). Persoonia 23:48–54. https://doi.org/10.3767/003158509x472187
Zhang YD, Ma J, Ma LG, Zhang XG (2010) Two new species of Kylindria from Fujian, China. Mycotaxon 114:367–371. https://doi.org/10.5248/114.367
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012a) Pleosporales. Fungal Divers 53:1–221. https://doi.org/10.1007/s13225-011-0117-x
Zhang H, Hyde KD, Mckenzie EHC, Bahkali AH, Zhou DQ (2012b) Sequence data reveals phylogenetic affinities of Acrocalymma aquatica sp. nov., Aquasubmersa mircensis gen. et sp. nov. and Clohesyomyces aquaticus (freshwater coelomycetes). Cryptogam Mycol 33:333–346. https://doi.org/10.7872/crym.v33.iss3.2012.333
Zhang H, Hyde KD, Abdel-Wahab MA, Abdel-Aziz FA, Ariyawansa HA, Ko TWK, Zhao RL, Alias SA, Bahkali AH, Zhou D (2013) A modern concept for Helicascus with a Pleurophomopsis-like asexual state. Sydowia 65:147–166. https://doi.org/10.1016/j.riam.2013.01.005
Zhang C, Jiang Z, Mahippong W, Pu J, Lü Y (2014a) Karst topography and hydro-geochemical characteristics in western Thailand and their correlation to that in southwestern China. Carsologica Sinica 33(1):1–8
Zhang Y, Liu YJ, Zhou YP, Zhang XD, Cui BK, He SH, Fournier J (2014b) Helicascus gallicus sp. nov., a new freshwater pleosporalean ascomycete from France. Phytotaxa 183:183–192. https://doi.org/10.11646/phytotaxa.183.3.5
Zhang JQ, Zhou YP, Dou ZP, Fournier J, Zhang Y (2015) Helicascus unilocularis sp. nov., a new freshwater pleosporalean ascomycete from the Caribbean area. Mycol Prog 14:1–8. https://doi.org/10.1007/s11557-015-1070-y
Zhang H, Dong W, Hyde KD, Bahkali AH, Liu JK, Zhou DQ, Zhang D (2016) Molecular data shows Didymella aptrootii is a new genus in Bambusicolaceae. Phytotaxa 247:99–108. https://doi.org/10.11646/phytotaxa.247.2.1
Zhang JF, Liu JK, Hyde KD, Yang W, Liu ZY (2017a) Fungi from Asian Karst formations II. Two new species of Occultibambusa (Occultibambusaceae, Dothideomycetes) from karst landforms of China. Mycosphere 8:550–559. https://doi.org/10.5943/mycosphere/8/4/4
Zhang H, Dong W, Hyde KD, Maharachchikumbura SSN, Hongsanan S, Bhat DJ, Al-Sadi AM, Zhang D (2017b) Towards a natural classification of Annulatascaceae-like taxa: introducing Atractosporales ord. nov. and six new families. Fungal Divers 85:75–110. https://doi.org/10.1007/s13225-017-0387-z
Zhang SN, Abdel-Wahab MA, Jones EBG, Hyde KD, Liu JK (2019) Additions to the genus Savoryella (Savoryellaceae), with the asexual morphs Savoryella nypae comb. nov. and S. sarushimana sp. nov. Phytotaxa 408:195–207. https://doi.org/10.11646/phytotaxa.408.3.4
Zhang J, Phookamsak R, Mapook A, Lu Y, Lv M (2021) Monilochaetes pteridophytophila (Australiascaceae, Glomerellales), a new fungus from tree fern. Biodivers Data J 9:e67248. https://doi.org/10.3897/bdj.9.e67248
Zhao GZ, Liu XZ (2005a) A review of Cirrenalia (hyphomycetes) and a new species. Fung Divers 18:201–209
Zhao GZ, Zhang TY (2005b) Notes on dictyosporic hyphomycetes from China. II. The genus Oncopodiella. Nova Hedwig 81:421–430. https://doi.org/10.1127/0029-5035/2005/0081-0421
Zhao GZ, Liu X, Wu W (2007) Helicosporous hyphomycetes from China. Fungal Divers 26:313–524
Zhao GZ, Cao AX, Zhang TY, Liu XZ (2011) Acrodictys (Hyphomycetes) and related genera from China. Mycol Prog 10:67–83. https://doi.org/10.1007/s11557-010-0677-2
Zhou SX, Qiao LJ, Kang JC, Hyde KD, Ma XY (2017) A new species of Monilochaetes from Nothapodytes pittosporoides. Phytotaxa 326(2):129–136. https://doi.org/10.11646/phytotaxa.326.2.4
Acknowledgements
This study was funded by The Research of Featured Microbial Resources and Diversity Investigation in the Southwest Karst area (Project no. 2014FY120100) and Thailand Research Fund, Grant RDG6130001, titled “Impact of climate change on fungal diversity and biogeography in the Greater Mekong Subregion”. Jian-Kui Liu thanks the Joint Fund of the National Natural Science Foundation of China and the Karst Science Research Center of Guizhou province (Grant No. U1812401). E.B. Gareth Jones is supported by the Distinguished Scientist Fellowship Program (DSFP), King Saud University, Saudi Arabia. Kevin D. Hyde would like to thank the National Research Council of Thailand (NRCT) Grant “Total fungal diversity in a given forest area with implications towards species numbers, chemical diversity and biotechnology” (Grant no. N42A650547). We are grateful to Prof. D. Jayarama Bhat for his valuable help of description corrections. Dr. Shaun Pennycook is thanked for his suggestion on fungal names and an essential nomenclatural review.
Funding
Funding was provided by Ministry of Science and Technology of the People's Republic of China (Grant no. 2014FY120100), Joint Fund of the National Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province (Grant no. U1812401).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Chayanard Phukhamsakda.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Liu, LL., Jones, E.B.G. et al. Freshwater fungi from karst landscapes in China and Thailand. Fungal Diversity 119, 1–212 (2023). https://doi.org/10.1007/s13225-023-00514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-023-00514-7