Abstract
Background
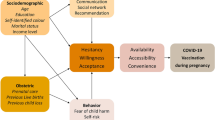
COVID-19 vaccines are safe in pregnancy, as they do not contain a live attenuated virus. Mass vaccination is a key to control the pandemic. Neonates have been shown to be susceptible to severe illness, so maternal vaccination is important to provide neonatal vaccination.
Methods
The present study was conducted for a period of one year from November 21, 2021 to October 2O, 2022 at the Department of Obstetrics and Gynecology A.S.J.S.A.T.D.S. medical college, Fatehpur. It was a hospital-based cross-sectional study. This study aimed to investigate the efficacy, safety, attitude, side effect and maternal neonatal outcome of COVID-19 vaccination among pregnant women.
Results
Out of 3320 pregnant women delivered, only 1170 (35.24%) received at least one dose of COVID-19 vaccine. 69.23% were unaware of the type of COVID-19 vaccine. 66.15% were vaccinated for both the doses before pregnancy. 12.30% of women had taken only the first dose of COVID vaccine before pregnancy. Majority had fever with chills after the first dose. Fatigue was most common side effect after second dose, and no one had any rash or allergic reaction. 56.15% delivered vaginally, 37.69% had LSCS for different obstetric indications, and 6.15% had instrumental delivery. During the antenatal period, 38.46% developed anemia, 11.54% had preterm labor, 2.05% had gestational diabetes, 2.30% developed preeclampsia, and 3.85% developed hypothyroidism. 3.07% prolonged labor in intrapartum period, and 6.92% women developed PPH. 50.77% newborns were between 2.5 and 2.9 kg, and majority 71.54% newborns had an APGAR score of 7 or more at 5 minutes. 14.62% newborns had meconium aspiration syndrome, 3.84% had respiratory distress syndrome, and 20.34% needed NICU admission more than 24 hours.
Conclusion
Available data do not support increased risk of adverse outcome following COVID-19 vaccination. We recommend vaccination during pregnancy as benefit outweigh the potential risk.
Similar content being viewed by others
References
Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–60.
Zambrano LD, Ellington S, Strid P, CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team, et al. Update: characteristics of symptomatic women of reproductive age with laboratory- confirmed SARS-CoV-2 infection by pregnancy status – United States, January 22–October 3, 2020. MMWR Morb Mortal WklyRep. 2020;69:1641–7. https://doi.org/10.1585/mmwr.mm6944e3.
Badr DA, Mattern J, Carlin A. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am J ObstetGynecol. 2020;223:764–8.
Viana J, vanDorp CH, Nunes A, et al. Con- trolling thepandemicduringtheSARS-CoV-2 vaccination rollout. Nat Commun. 2021;12:3674.
Kalafat E, O’Brien P, Heath PT, et al. Benefits and potentialharmsofCOVID-19vaccination during pregnancy: evidence summary for patient counseling. Ultrasound Obstet Gynecol. 2021;57:681–6.
Federation of Obstetric and Gynecological Societies of India. FOGSI Position Statement: COVID Vaccination for Pregnant and Breastfeeding Women. https://www.fogsi.org/wp-content/uploads/covid19/fogsi-statement-on-covid-vaccination-in-pregnancy-and-bf.pdf.
Donders GGG, Grinceviciene S, Haldre K, On The Behalf Of The Covid-Isidog Guideline Group, et al. ISIDOG consensus guidelines on COVID-19 vaccination for women before, during and after pregnancy. J Clin Med. 2021;10:2902. https://doi.org/10.3390/jcm10132902.
Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J ObstetGynecol. 2022;226(236):e1-14.
Kumari A, Kumari S, Kujur M, et al. Acceptance rate of COVID-19 vaccine and its determinants among indian pregnant women: a hospital-based cross-sectional analysis. Cureus. 2022;14(10): e30682. https://doi.org/10.7759/cureus.30682.PMID:36439607;PMCID:PMC9691374.
Dhakal R, Shapkota S, Shrestha P, et al. Pregnant women’s awareness, perception, and acceptability of COVID-19 vaccine attending antenatal clinics in Bharatpur. Nepal PLoS One. 2023;18(3): e0278694. https://doi.org/10.1371/journal.pone.0278694.PMID:36920992;PMCID:PMC10016669.
Theiler RN, Wick M, Mehta R, et al. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. https://doi.org/10.1016/j.ajogmf.2021.100467.
Ghamri RA, Othman SS, Alhiniah MH, et al. Acceptance of COVID-19 vaccine and associated factors among pregnant women in Saudi Arabia. Patient Prefer Adherence. 2022;16:861–73.
Operational guidance for COVID-19 vaccination of pregnant women. Ministry of Health and Family Welfare; 2021. Published, https://www.mohfw.gov.in/pdf/OperationalGuidanceforCOVID19vaccinationofPregnantWoman.pdf. [Accessed 2 July 2021].
Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;S0002–9378(21):00187–93. https://doi.org/10.1016/j.ajog.2021.03.023. (PMID: 33775692).
Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326(8):728–35. https://doi.org/10.1001/jama.2021.11035.PMID:34251417;PMCID:PMC8276131.
Villar J, Soto Conti CP, Gunier RB, INTERCOVID-2022 International Consortium, et al. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet. 2023;401(10375):447–57. https://doi.org/10.1016/S0140-6736(22)02467-9.
Rottenstreich M, Sela HY, Rotem R, et al. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129:248–55.
Peretz-Machluf R, Hirsh-Yechezkel G, Zaslavsky-Paltiel I, et al. Obstetric and neonatal outcomes following COVID-19 vaccination in pregnancy. J Clin Med. 2022;11(9):2540. https://doi.org/10.3390/jcm11092540.PMID:35566665;PMCID:PMC9105434.
Prasad S, Kalafat E, Blakeway H, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun. 2022;13(1):2414. https://doi.org/10.1038/s41467-022-30052-w.PMID:35538060;PMCID:PMC9090726.
Beharier O, Plitman Mayo R, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131(13): e150319. https://doi.org/10.1172/JCI150319.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of institution which complies with the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
All authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pragya shree is a Associate Professor; Vandana Verma is a Associate Professor; Neetika Patel is a Senior Resident; Roshani Gupta is a Assistant Professor; Kamayni Yadav is a Senior Resident.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
shree, P., Verma, V., Patel, N. et al. Awareness and Safety of COVID-19 Vaccination in Pregnancy. J Obstet Gynecol India (2024). https://doi.org/10.1007/s13224-023-01918-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13224-023-01918-w