Abstract
Purpose
Gynecological cancers are common neoplasms in clinical settings with a high impact on the economy of communities. The medical literature is an essential resource to guide clinical decision-making, and misconduct in researches undermines the credibility and integrity of research in general. We aimed to evaluate the quality of Cochrane gynecological cancers reviews and their understudies RCTS among the different biases dimensions.
Methods
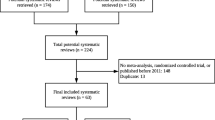
This cross-sectional analytical study was performed on 118 systematic reviews published by the Cochrane gynecological cancers Group up to June 2021. The risk of bias was assessed in each Cochrane survey using the Joanna Bridges Institute (JBI) critical assessment tool consisting of 11 questions. The JBI checklist for systematic reviews and research syntheses is available at https://jbi.global/critical-appraisal-tools. After a systematic critical evaluation of the reviews and meta-analysis, we extracted a different bias from all of their understudied RCTs examined in these systematic reviews, which were evaluated by systematic review authors using a standard bias risk tool developed by the Cochrane Group.
Results
Cochrane gynecological cancers reviews had high quality based on appraise results using the JBI appraisal checklist. In addition, all of the included studies used PRISMA standards for reporting their results. However, in their understudied RCTs, the most prevalent risk of bias was unclear selection bias (allocation concealment) and performance bias (blinding of participants and personnel). Also, the highest risk of bias was blinding participants and personnel (performance bias) and incomplete outcome data (attrition bias). Our results showed that the lowest risk of bias was incomplete outcome data (attrition bias) and random sequence generation (selection bias).
Conclusion
Although most Cochrane gynecological cancers reviews had high quality, unclear performance bias was the highest in their understudied RCTs, indicating structural deficiencies.


Similar content being viewed by others
1. References
Bhatt A. Quality of clinical trials: a moving target. Perspect Clin Res. 2011;2(4):124–8.
Larsen PO, von Ins M. The rate of growth in scientific publication and the decline in coverage provided by science citation index. Scientometrics. 2010;84(3):575–603.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: Cancer J Clin. 2019;69(1):7–34.
Lim MC, Won YJ, Ko MJ, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999–2015. J Gynecol Oncol. 2019;30(1):e38.
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71(3):209–49.
Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–68.
Porritt K, Gomersall J, Lockwood C. JBI’s systematic reviews: study selection and critical appraisal. Am J Nurs. 2014;114(6):47–52.
Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40.
Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;18:343.
Rosenstein AH, O’Daniel M. Disruptive behavior and clinical outcomes: perceptions of nurses and physicians: nurses, physicians, and administrators say that clinicians’ disruptive behavior has negative effects on clinical outcomes. AJN Am J Nurs. 2005;105(1):54–64.
Khorsan R, Crawford C. How to assess the external validity and model validity of therapeutic trials: a conceptual approach to systematic review methodology. Evid Based Complement Alternat Med. 2014.
Rosner AL. Evidence-based medicine: revisiting the pyramid of priorities. J Bodyw Mov Ther. 2012;16(1):42–9.
Rudmik LR, Walen SG, Dixon E, et al. Evaluation of meta-analyses in the otolaryngological literature. Otolaryngol-Head Neck Surg. 2008;139(2):187–94.
Junhua Z, Hongcai S, Xiumei G, et al. Methodology and reporting quality of systematic review/meta-analysis of traditional Chinese medicine. J Altern Complement Med. 2007;13(8):797–806.
Gagnier JJ, Kellam PJ. Reporting and methodological quality of systematic reviews in the orthopaedic literature. J Bone Jt Surg Am. 2013;95(11):e771–7.
Goenka L, Rajendran S, Arumugam K, et al. The assessment of the quality of randomized controlled trials published in Indian medical journals. Perspect Clin Res. 2019;10(2):79–83.
Liu X, Rivera SC, Moher D, et al. SPIRIT-AI and CONSORT-AI working group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI Extension. BMJ. 2020;9(370):m3164.
Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126(2):619.
Acknowledgements
We would like to thank Tabriz University of Medical Sciences, Tabriz, Iran for financial support.
Funding
This study is funded by Tabriz University of Medical Sciences (Grant NO. 64482).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Ethical Approval
Local Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran approved the proposal: ID IRTBZMED.REC.1396.774.
Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Local Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (ID IRTBZMED.REC.1396.774).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hajebrahimi, S., Dalir Akbari, N., Haji Kamanaj, A. et al. Quality of the Systematic Reviews in Cochrane Gynecological Cancer Group and Their Understudied RCTs. J Obstet Gynecol India 72 (Suppl 1), 346–351 (2022). https://doi.org/10.1007/s13224-022-01655-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-022-01655-6