Abstract
Introduction
Iron deficiency anemia (IDA) in pregnancy has a prevalence as high as 40–60% in different countries of the world. Oral iron is used to treat his commonest medical disorder in pregnancy. Ferrous sulphate is associated with considerable side effects. Ferric carboxymaltose (FCM) is a newer iron preparation which allows for single and higher dose (up to 1000 mg) of IV iron infusion. This study was conducted to compare the efficacy of FCM and FS in treating IDA during pregnancy.
Methods
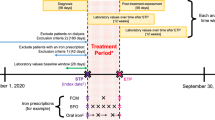
A randomised control trial was done at a tertiary care centres involving 362 women (181 women each in FS and FCM group). The pregnant anemic women with IDA were enrolled between 18 and 34 weeks of pregnancy. They were given 1000 mg of FCM iv as single dose or were given FS tablets twice daily (120 mg iron daily). The data were collected for rise in the Hb and serum ferritin over a period of 6 weeks.
Results
Nine and 18 patients were lost to follow-up in the FCM and FS group, respectively. The data were analysed as per protocol analysis. FCM group women showed 2.6 gm% rise in Hb compared to 1.7 gm% of FS group. One hundred and sixty-six out of 172 women in FS group achieved anemia correction at 6 weeks. No difference was observed in the neonatal outcome. No major side effects were observed in the either group.
Conclusion
In our study, FCM was more effective than oral FS in increasing Hb in women with IDA during pregnancy. This clinical benefit with FCM was achieved without the concerns for safety and tolerability of the drug.


Similar content being viewed by others
References
Balarajan Y, Ramakrishnan U, Ozaltin E, et al. Anaemia in low-income and middle-income countries. Lancet. 2011;378(9809):2123–35.
WHO. The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015.
Qassim A, Mol BW, Grivell RM, et al. Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: a systematic review. Aust N Z J Obstet Gynaecol. 2018;58(1):22–39.
Nair M, Churchill D, Robinson S, et al. Association between maternal haemoglobin and stillbirth: a cohort study among a multi-ethnic population in England. Br J Haematol. 2017;179:829–37.
Toteja GS, Singh P, Dhillon BS, et al. Prevalence of anemia among pregnant women and adolescent girls in 16 districts of India. Food Nutr Bull. 2006;27(4):311–5.
Rai RK, Fawzi WW, Barik A, et al. The burden of iron-deficiency anaemia among women in India: how have iron and folic acid interventions fared? WHO South East Asia J Public Health. 2018;7:18–23.
Breymann C, Milman N, Mezzacasa A, FER-ASAP Investigators, et al. Ferric carboxymaltose vs. oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP). J Perinat Med. 2017;45(4):443–53.
Mwangi MN, Mzembe G, Moya E, et al. Protocol for a multicentre, parallel-group, open-label randomised controlled trial comparing ferric carboxymaltose with the standard of care in anaemic Malawian pregnant women: the REVAMP trial. BMJ Open. 2021;11:e053288.
Jose A, Mahey R, Sharma JB, et al. Comparison of ferric Carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy-randomised controlled trial. BMC Pregnancy Childb. 2019;19:54.
Froessler B, Gajic T, Dekker G, et al. Treatment of iron deficiency and iron deficiency anemia with intravenous ferric carboxymaltose in pregnancy. Arch Gynecol Obstet. 2018;298:75–82.
DeLoughery TG. Safety of oral and intravenous iron. Acta Haematol. 2019;142:8–12.
Pels A, Ganzevoort W. Safety and efficacy of ferric carboxymaltose in anemic pregnant women: a retrospective case control study. Obstet Gynecol Int. 2015;2015:728952.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study concept: SC and AS. Study conduct: SC, AS, AHA, and DJ. Drafting and manuscript revision: SC and AS. Statistical analysis: AS, CHA, and DJ. Study supervision: SC.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the institutional ethics committee at AFMC, Pune, and I certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chawla, S., Singh, A., Jhamb, D. et al. A Randomised Controlled Trial to Compare Injection Ferric Carboxymaltose and Oral Iron in Treating Iron Deficiency Anemia During Pregnancy. J Obstet Gynecol India 72, 492–496 (2022). https://doi.org/10.1007/s13224-022-01653-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-022-01653-8