Abstract
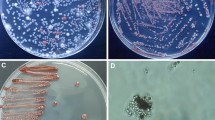
Microbial extracts continue to be a productive source of new molecules with biotechnological importance. Fungi of the genus Penicillium are known to produce biologically active secondary metabolites. The goal of this work is verify the production of antimicrobial metabolites by Penicillium chrysogenum IFL1 using agro-industrial residues. P. chrysogenum IFL1 produced active metabolites growing on the agro-industrial residues, grape waste and cheese whey. The 7-day cultures showed antimicrobial activities against bacteria, fungi and amoebae. The filtrate of the cheese whey culture inhibited the growth of the bacteria Staphylococcus aureus, Bacillus cereus and Pseudomonas aeruginosa, the fungus Fusarium oxysporum and the amoeba Acanthamoeba polyphaga. Due to the greater antimicrobial activity of the cheese whey culture, a footprinting profile was carried out using the ESI-MS and ESI-MS/MS techniques. The presence of penicillin G and other metabolites that have antimicrobial activity such as penicillin V and rugulosin can be suggested. P. chrysogenum IFL1 was able to produce a wide variety of antimicrobial compounds on agro-industrial residues, which makes the process ecologically friendly.



Similar content being viewed by others
References
Barkai-Golan R (2008) Penicillium mycotoxins. In: Barkai-Golan R, Paster N (eds) Mycotoxins in fruits and vegetables. Elsevier, New York, pp 153–183
Barrios-Gonzalez J, Tomasini A, Viniegra-Gonzalez G, Lopez L (1988) Penicillin production by solid-state fermentation. Biotechnol Lett 10:793–798
Becker-Ritt AB, Martinelli AHS, Mitidieri S, Feder V, Wassermann GE, Santi L, Vainstein MH, Oliveira JTA, Fiuza LM, Pasquali G, Carlini CR (2007) Antifungal activity of plant and bacterial ureases. Toxicon 50:971–983
Benitez LB, Caumo K, Brandelli A, Rott MB (2010) Bacteriocin-like substance from Bacillus amyloliquefaciens shows remarkable inhibition of Acanthamoeba polyphaga. Parasitol Res 108:687–691
Bi P, Li D, Dong H (2009) A novel technique for the separation and concentration of penicillin G from fermentation broth: aqueous two-phase flotation. Sep Purif Technol 69:205–209
Blumenthal CZ (2004) Production of toxic metabolites in Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei: justification of mycotoxin testing in food grade enzyme preparations derived from the three fungi. Regul Toxicol Pharmacol 39:214–228
Boonman N, Wiyakrutta S, Sriubolmas N, Chusattayanond AD (2008) Acanthamoebicidal activity of Fusarium sp. Tlau 3, and endophytic fungus from Thunbergia laurifolia Lindl. Parasitol Res 103:1083–1090
Brunati M, Rojas JL, Sponga F, Ciciliato I, Losi D, Göttlich E, Hoog S, Genilloud O, Marinelli F (2009) Diversity and pharmaceutical screening of fungi from benthic mats of Antarctic lakes. Mar Genomics 2:43–50
Chomicz L, Padzik M, Graczyk Z, Starosciak B, Graczyk TK, Naprawska A, Oledzka G, Szostakowska B (2010) Acanthamoeba castellanii: In vitro effects of selected biological, physical and chemical factors. Exp Parasitol 126:103–105
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20
Derda M, Hadas E, Thiem B (2009) Plant extracts as natural amoebicidal agents. Parasitol Res 104:705–708
Edel V, Steinberg C, Gautheron N, Recorbet G, Alabouvette C (2001) Genetic diversity of Fusarium oxysporum populations isolated from different soils in France. FEMS Microbiol Ecol 36:61–71
El-Enshasy HA (2007) Filamentous fungal cultures - Process characteristics, products, and applications. In: Yang ST (ed) Bioprocessing for value-added products from renewable resources. Elsevier, New York, pp 225–261
Frisvad JC, Smedsgaard J, Larsen TO, Samson RA (2004) Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud Mycol 49:201–241
Goodacre R, Trew S, Wrigley-Jones C, Saunders G, Neal MJ, Porter N, Kell DB (1995) Rapid and quantitative analysis of metabolites in fermentor broths using pyrolysis mass spectrometry with supervised learning: application to the screening of Penicillium chrysogenum fermentations for the overproduction of penicillins. Anal Chim Acta 313:25–43
Goze I, Alim A, Dag S, Tepe B, Polat ZA (2009) In vitro amoebicidal activity of Salvia staminea and Salvia caespitosa on Acanthamoeba castellani and their cytotoxic potentials on corneal cells. J Ocul Pharmacol Ther 25:293–298
Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15:639–652
Kanafani ZA, Fowler VG (2006) Staphylococcus aureus infections: New challenges, from an old pathogen. Enferm Infect Microbiol Clin 24:82–193
Kerr KG, Snelling AM (2009) Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect 73:338–344
Lopes FC, Silva LAD, Tichota DM, Daroit DJ, Velho RV, Pereira JQ, Côrrea APF, Brandelli A (2011) Production of proteolytic enzymes by a keratin-degrading Aspergillus niger. Enzyme Res 2011:487093. doi:10.4061/2011/487093
Mandal S, Mallick N, Mitra A (2009) Salicylic acid-induced resistance to Fusarium oxysporum f. sp. lycopersici in tomato. Plant Physiol Biochem 47:642–649
Mandenius CF, Brundin A (2008) Bioprocess Optimization Using Design-of-Experiments Methodology. Biotechnol Prog 24:1191–1203
Martínez-Blanch JF, Sánchez G, Aznar R (2009) Development of a real-time PCR assay for detection and quantification of enterotoxigenic members of Bacillus cereus group in food samples. Int J Food Microbiol 135:15–21
Monaci L, Aresta A, Palmisano F, Visconti A, Zambonin CG (2002) Amino-bonded silica as stationary phase for liquid chromatographic determination of cyclopiazonic acid in fungal extracts. J Chromatogr A 955:79–86
Motta AS, Brandelli A (2002) Characterization of an antibacterial peptide produced by Brevibacterium linens. J Appl Microbiol 92:63–71
Nielsen J (1997) Physiological Engineering. In: Nielsen J (ed) Physiological Engineering Aspects of Penicillium chrysogenum. World Scientific, Singapore, pp 23–54
Nielsen KF, Smedsgaard J (2003) Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardized liquid chromatography-UV-mass spectrometry methodology. J Chromatogr A 1002:111–136
Nigam PS (2009) Production of bioactive secondary metabolites. In: Nigam PS, Pandey A (eds) Biotechnology for agro-industrial residues utilization. Springer, New York, pp 129–146
Nigam PS, Gupta N, Anthwal A (2009) Pre-treatment of agro-industrial residues. In: Nigam PS, Pandey A (eds) Biotechnology for agro-industrial residues utilization. Springer, New York, pp 13–36
Pérez-Garcia A, Romero D, Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol 22:1–7
Petit P, Lucas EMF, Abreu LM, Pfenning LH, Takahashi JA (2009) Novel antimicrobial secondary metabolites from a Penicillium sp. isolated from Brazilian cerrado soil. Electron J Biotechnol 4:1–9
Pimenta EF, Vita-Marques AM, Tininis A, Seleghim MHR, Sette LD, Veloso K, Ferreira AG, Williams DE, Patrick BO, Dalisay DS, Andersen RJ, Berlinck RGS (2010) Use of experimental design for the optimization of the production of new secondary metabolites by two Penicillium species. J Nat Prod 73:1821–1832
Pitt JI, Hocking AD (2009) Fungi and food spoilage. Springer, New York
Polat ZA, Vural A, Ozan F, Tepe B, Özcelik S, Cetin A (2008) In vitro evaluation of the amoebicidal activity of garlic (Allium sativum) extract on Acanthamoeba castellanii and its cytotoxic potential on corneal cells. J Ocul Pharmacol Ther 24:8–14
Poole K (2001) Multidrug resistance in Gram-negative bacteria. Curr Opin Microbiol 4:500–508
Rancic A, Sokovic M, Karioti A, Vukojevic J, Skaltsa H (2006) Isolation and structural elucidation of two secondary metabolites from the filamentous fungus Penicillium ochrochloron with antimicrobial activity. Environ Toxicol Pharmacol 22:80–84
Ródio C, Vianna DR, Kowalski KP, Panatieri LF, von Poser G, Rott MB (2008) In vitro evaluation of the amebicidal activity of Pterocaulon polystachyum (Asteraceae) against trophozoites of Acanthamoeba castellanii. Parasitol Res 104:191–194
Rouse S, Harnett D, Vaughan A, van Sinderen D (2008) Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J Appl Microbiol 104:915–923
Rundberget T, Skaar I, Flaoyen A (2004) The presence of Penicillium and Penicillium mycotoxins in food wastes. Int J Food Microbiol 90:181–188
Senyuva HZ, Gilbert J, Öztürkoglu S (2008) Rapid analysis of fungal cultures and dried figs for secondary metabolites by LC/TOF-MS. Anal Chim Acta 617:97–106
Serra AT, Matias AA, Nunes AVM, Leitão MC, Brito D, Bronze R, Silva S, Pires A, Crespo MT, San Romão MV, Duarte CM (2008) In vitro evaluation of olive- and grape-based natural extracts as potential preservatives for food. Innov Food Sci Emerg Technol 9:311–319
Skouri-Gargouri H, Ali BM, Gargouri A (2009) Molecular cloning, structural analysis and modeling of the AcAFP antifungal peptide from Aspergillus clavatus. Peptides 30:1798–1804
Smedsgaard J, Hansen ME, Frisvad JC (2004) Classification of terverticillate Penicillia by electrospray mass spectrometric profiling. Stud Mycol 49:243–251
Song J (2003) Introduction: the goals of antimicrobial therapy. Int J Infect Dis 7:51–54
Sumarah MW, Adams GW, Berghout J, Slack GJ, Wilson AM, Miller JD (2008) Spread and persistence of a rugulosin-producing endophyte in Picea glauca seedlings. Mycol Res 112:731–736
Tatsuta K, Yamaguchi T (2005) The first stereoselective total synthesis of antiviral antibiotic, xanthocillin X dimethylether and its stereoisomer. Tetrahedr Lett 46:5017–5020
Vishwanath V, Sulyok M, Labuda R, Bicker W, Krska R (2009) Simultaneous determination of 186 fungal and bacterial metabolites in indoor matrices by liquid chromatography/ tandem mass spectrometry. Anal Bioanal Chem 395:1355–1372
Wang SL, Yen YH, Tsiao WJ, Chang WT, Wang CL (2002) Production of antimicrobial compounds by Monascus purpureus CCRC31499 using shrimp and crab shell powder as a carbon source. Enzyme Microb Technol 31:337–344
WHO - World Health Organization - The top 10 causes of death http://www.who.int/mediacentre/factsheets/fs310/en/index.html. Acessed on July 29th, 2012.
Acknowledgments
Authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support. In addition, we also thank Dr. Patricia Valente da Silva, Dr. Charley Christian Staats, Dr. Fernanda Stanisçuaski (UFRGS) and Dr. João Lúcio de Azevedo (ESALQ/USP) for the insightful suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopes, F.C., Tichota, D.M., Sauter, I.P. et al. Active metabolites produced by Penicillium chrysogenum IFL1 growing on agro-industrial residues. Ann Microbiol 63, 771–778 (2013). https://doi.org/10.1007/s13213-012-0532-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-012-0532-6