Abstract
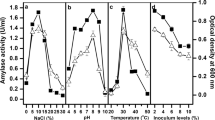
The purpose of this study was to determine the influence of growth conditions and medium composition on the thermostable, Ca+2-independent, maltose producing α-amylase production in submerged fermentation (SmF) by Streptomyces sp. MSC702—a strain newly isolated from mushroom compost. Production of α-amylase enzyme by a Streptomyces strain was detected on semi-synthetic media containing rice bran and wheat bran in a 1:2 ratio as the sole carbon source. The effect on α-amylase production of different medium ingredients and additives, such as NaCl, additional carbon sources, nitrogen sources, metal salts, nucleotides, phytohormones, surfactants and other chemicals, was examined. NaCl played no notable role in the biosynthesis of α-amylase, with the strain being able to grow at 14% (w/v) NaCl with ~76% α-amylase activity. Among the different cations tested, Hg2+ showed no inhibitory effect on α-amylase production. The best combination of physical parameters for the maximum production of α-amylase was a temperature of 50°C, pH 7.0 and an incubation period of 48 h. The best combination of chemical parameters with rice bran 1.0% (w/v) and wheat bran 2.0% (w/v) was dipotassium hydrogen phosphate 0.1% (w/v), magnesium sulfate 0.1% (w/v) with d-inositol (as additional carbon source) 1.0% (w/v), peptone (as nitrogen source) 0.1% (w/v), manganese sulphate (as additional metal ion) 0.05% (w/v) and Tween-80 (as surfactant) 1.0% (v/v). With the application of these culture conditions, α-amylase production increased by ~1.42 fold.





Similar content being viewed by others
References
Abou-Elela GM, Nermeen AE, Wefky SH (2009) Statistical optimization of cold adapted α-amylase production by free and immobilized cells of Nocardiopsis aegyptia. J Appl Sci Res 5(3):286–292
Ammar YB, Matsubara T, Ito K, Iizuka M, Limpaseni T, Pongsawasdi P, Minamiura N (2002) New action pattern of a maltose-forming α-amylase from Streptomyces sp. and its possible application in bakery. J Biochem Mol Biol 35(6):568–575
Arnesen S, Eriksen SH, Olsen J, Jensen B (1998) Increased production of α-amylase from Thermomyces lanuginosus by the addition of Tween 80. Enzyme Microb Technol 23:249–252
Arrieta-Escobar A, Belin JM (1982) Effects of polyphenolic compounds on the growth and cellulolytic activity of a strain of Trichoderma viride. Biotechnol Bioeng 24:983–989
Babu KR, Satyanarayana T (1993) Parametric optimization of extracellular α-amylase production by thermophilic Bacillus coagulans. Folia Microbiol 38:77–80
Bernharsdotter ECMJ, Ng JD, Garriott OK, Pusey ML (2005) Enzymic properties of an alkaline chelator-resistant α-amylase from an alkaliphilic Bacillus sp. isolate L1711. Process Biochem 40:2401–2408
Bernier R, Stutzenberger F (1987) Preferential uptake of cellobiose by Thermomonospora curvata. Appl Environ Microbiol 53:1743–1747
Bruinenberg PM, Hulst AC, Faber A, Voogd RH (1996) A process for surface sizing or coating of paper. European Patent Application EP 0,690,170 A1
Burhan A, Nisa U, Gokhan C, Omer C, Ashabil A, Osman G (2003) Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem 38:1397–1403
Busch JE, Stutzenberger FJ (1997) Amylolytic activity of Thermomonospora fusca. World J Microbiol Biotechnol 13:637–642
Chakraborty S, Khopade A, Kokare C, Mahadik K, Chopade B (2009) Isolation and characterization of novel α-amylase from marine Streptomyces sp. D1. J Mol Catal B Enzym 58:17–23
Collins BS, Kelly CT, Fogarty WM, Doyle EM (1993) The high maltose producing α-amylase of the thermophilic actinomycete, Thermomonospora curvata. Appl Microbiol Biotechnol 39:31–35
Costa AM, Ribeiro WX, Kato E, Monteiro ARG, Peralta RM (2008) Production of tannase by Aspergillus tamarii in submerged cultures. Braz Arch Biol Technol 51(2):399–404
Dey G, Mitra A, Banerjee R, Maiti BR (2001) Enhanced production of amylase by optimization of nutritional constituents using response surface methodology. J Biochem Eng 7:227–231
Doyle EM, Kelly CT, Fogarty WM (1989) The high maltose-producing α-amylase of Penicillium expansum. Appl Microbiol Biotechnol 30:492–496
Egas MCV, da Costa MS, Cowan DA, Pires EMV (1998) Extracellular α-amylase from Thermus filiformis Ork A2: purification and biochemical characterization. Extremophiles 2:23–32
Gemishev OT, Vaseva II, Atev AP (2005) Abscisic acid and ethylene influence on endo-1,4-β-glucanase activity in Trichoderma reesei I-27. Biotechnol Biotechnol Eq 19(3):106–112
Gigras P, Sahai V, Gupta R (2002) Statistical media optimization and production of ITS α-amylase from Aspergillus oryzae in a bioreactor. Curr Microbiol 45:203–208
Goldberg JD, Edwards C (1990) Purification and characterization of an extracellular amylase from a thermophilic streptomycete. Appl Bacteriol 69:712–717
Goyal N, Gupta JK, Soni SK (2005) A novel raw starch digesting thermostable α-amylase from Bacillus sp. I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme Microb Technol 37:723–734
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial α-amylases: a biotechnological perspective. Process Biochem 38:1599–1616
Haki GD, Rakshit SK (2003) Developments in industrially important thermostable enzymes: a review. Bioresour Technol 89:17–34
Hamilton LM, Kelly CT, Fogarty WM (1999) Production and properties of the raw starch-digesting α-amylase of Bacillus sp. IMD 435. Process Biochem 35:27–31
Hendriksen HV, Pedersen S, Bisgard-Frantzen H (1999) A process for textile warp sizing using enzymatically modified starches. Patent Application WO 99/35325
Hernandez MS, Rodriguez MR, Guerra NP, Roses RP (2006) Amylase production by Aspergillus niger in submerged cultivation on two wastes from food industries. J Food Eng 73:93–100
Ishii T, Shrestha YH, Matsumoto I, Kadoya K (1996) Effect of ethylene on the growth of vesicular-arbuscular mycorrhizal fungi and on the mycorrhizal formation of trifoliate orange roots. J Jpn Soc Hortic Sci 65(3):525–529
Kar S, Ray RC (2008) Partial characterization and optimization of extracellular thermostable Ca2+ inhibited α-amylase production by Streptomyces erumpens MTCC 7317. J Sci Ind Res 67:58–64
Kaur P, Satyanarayana T (2004) Production and saccharification by a thermostable and neutral glucoamylase of a thermophilic mould Thermomucor indicae-seudaticae. J Microbiol Biotechnol 20:419–425
Kelly CT, Giblin M, Forgarty WM (1986) Resolution, purification and characterization of two extracellular glucohydrolases, β-glucosidase and maltase of Bacillus licheniformis. Can J Microbiol 32:342–347
Kottwitz B, Upadek H, Carrer G (1994) Applications and benefits of enzymes in detergent. Chim Oggi 12:21–24
Kundu AK, Das S, Gupta TK (1973) Influence of culture and nutritional conditions on the production of amylase by the submerged culture of Aspergillus oryzae. J Ferment Technol 51:142–150
Kuo MJ, Hartman PA (1966) Isolation of amylolytic strains of Thermoactinomyces vulgaris and production of thermophilic actinomycete amylases. J Bacteriol 92:723–726
Lane RL, Williams RJ (1948) Inositol, an active constituent of pancreatic (alpha) amylase. Arch Biochem 19:329
Liu XD, Xu Y (2008) A novel raw starch digesting α-amylase from a newly isolated Bacillus sp. YX-1: purification and characterization. Bioresour Technol 99:4315–4320
Malhotra R, Noorvez SM, Satyanarayana T (2000) Production and partial characterization of thermostable and calcium independent alpha amylase of an extreme thermophile Bacillus thermooleovorans NP54. Lett Appl Microbiol 31:378–384
McMahon HEM, Kelly CT, Fogarty WM (1997) Effect of growth rate on α-amylase production by Streptomyces sp. IMD 2679. Appl Microbiol Biotechnol 48:504–509
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Najafi MF, Kembhavi A (2005) One-step purification and characterization of an extracellular α-amylase from marine Vibrio sp. Enzyme Microb Technol 36:535–539
Narayana KJP, Vijayalakshmi M (2008) Production of extracellular α-amylase by Streptomyces albidoflavus. Asian J Biochem 3(3):194–197
Newton GL, Bewley CA, Dwyer TJ, Horn R, Aharonowitz Y, Cohen G, Davies J, Faulkner DJ, Fahey RC (1995) The structure of U17 isolated from Streptomyces clavuligerus and its properties as an antioxidant thiol. Eur J Biochem 230:821–825
Obi SKC, Odibo FJC (1984) Some properties of a highly thermostable α-amylase from Thermoactinomyces sp. Can J Microbiol 30:780–785
Ozlem K, Ugur C, Burhan A (2005) Effects of carbon sources and various chemicals on the production of a novel amylase from a thermophilic Bacillus sp. K-12. Turk J Biol 29:99–103
Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, Mohan R (2000) Advances in microbial amylases. Biotechnol Appl Biochem 1:135–152
Parajo JC, Dominguez H, Dominguez JM (1998) Biotechnological production xylitol. Part 3: operation in culture media made from lignocellulose hydrolysates. Bioresour Technol 66:25–40
Pessoa A, Mancilha IMD, Sato S (1997) Evaluation of sugarcane hemicellulose hydrolysate for cultivation of yeasts and filamentous fungi. J Ind Microbiol Biotechnol 18:360–363
Polizeli MLTM, Jorge JA, Terenzi HF (1996) Effect of carbon source on the β-glucosidase system of the thermophilic fungus Humicola grisea. World J Microbiol Biotechnol 12:297–299
Prakash B, Vidyasagar M, Madhukumar MS, Muralikrishna G, Sreeramulu K (2009) Production, purification, and characterization of two extremely halotolerant, thermostable, and alkali-stable α-amylases from Chromohalobacter sp. TVSP 101. Process Biochem 44:210–215
Rawat M, Av-Gay Y (2007) Mycothiol-dependent proteins in actinomycetes. FEMS Microbiol 31(3):278–292
Saxena RK, Dutt K, Agarwal L, Nayyar P (2007) A highly thermostable and alkaline amylase from a Bacillus sp. PN5. Bioresour Technol 98:260–265
Shatta AM, El-hamahmy AF, Ahmed FA, Ibrahim MMK, Arafa AMI (1990) The influence of certain nutritional and environmental factors on the production of amylase enzyme by Streptomyces aureofaciens 77. J Islamic Acad Sci 3:134–138
Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A (2006) α-Amylases from microbial sources – an overview on recent developments. Food Technol Biotechnol 44(2):173–184
Srivastava RAK, Baruah JN (1986) Culture conditions for production of thermostable amylase by Bacillus stearothermophilus. Appl Environ Microbiol 52(1):179–184
Suzuki S, Yamamoto K, Okuda T, Nishio M, Nakanishi N, Komatsubara S (2000) Selective isolation and distribution of Actnomadura rugatobiospora strains in soil. Actinomycetologica 14:1–7
Techapun C, Sinsuwongwat S, Poosaran N, Watanabe M, Sasaki K (2002) Thermostable and alkaline tolerant cellulase - free xylanase produced by thermotolerant Streptomyces sp. Ab106. J Biosci Bioeng 93(4):431–433
Tengerdy RP, Szakacs G (2003) Bioconversion of lignocellulose in solid substrate fermentation. Biochem Eng J 13:169–179
Tonkova A (2006) Microbial starch converting enzymes of the α-amylase family. In: Ray RC, Wards OP (eds) Microbial biotechnology in horticulture, vol I. Science, Enfield, pp 421–472
Upton ME, Fogarty WM (1977) Production and purification of thermostable amylase and protease of Thermomonospora viridis. Appl Environ Microbiol 33(1):59–64
Vidal MEF, Vivas AF, Gonzalez F, Arias JM (1995) Properties and significance of an α-amylase produced Myxococcus coralloides. J Appl Bacteriol 78:14–19
Walsh G (2002) Industrial enzymes, an introduction. In: Walsh G (ed) Biochemistry and biotechnology. Wiley, New York, pp 393–454
Wu WX, Mabinadji J, Betrand TF, Wu WX (1999) Effect of culture conditions on the production of an extracellular thermostable alpha-amylase from an isolate of Bacillus sp. J Zhejiang Univ Agric Life Sci 25:404–408
Yang SS, Wang JY (1999) Protease and amylase production of Streptomyces rimosus in submerged and solid state cultivations. Bot Bull Acad Sin 40:259–265
Zahner H, Ettlinger L (1957) Zur Systematik der Actinomyceten. 3. Die Verwertung Vershiedener Kohlenstoffquellen als Hilfsmittel der Artbestimmung innerhalb der Gattung Streptomyces. Arch Mikrobiol 26:307–328
Acknowledgment
The authors are grateful to the University Grants Commission, Selection and Award Bureau, New Delhi, Government of India, for providing financial support to carry out this work and awarding a fellowship to R.S. under the scheme of ‘Rajiv Gandhi National Fellowship’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, R., Kapoor, V. & Kumar, V. Production of thermostable, Ca+2-independent, maltose producing α-amylase by Streptomyces sp. MSC702 (MTCC 10772) in submerged fermentation using agro-residues as sole carbon source. Ann Microbiol 62, 1003–1012 (2012). https://doi.org/10.1007/s13213-011-0340-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-011-0340-4